Paper of the Month - July 2024
selected by the BMAS Scientific Board
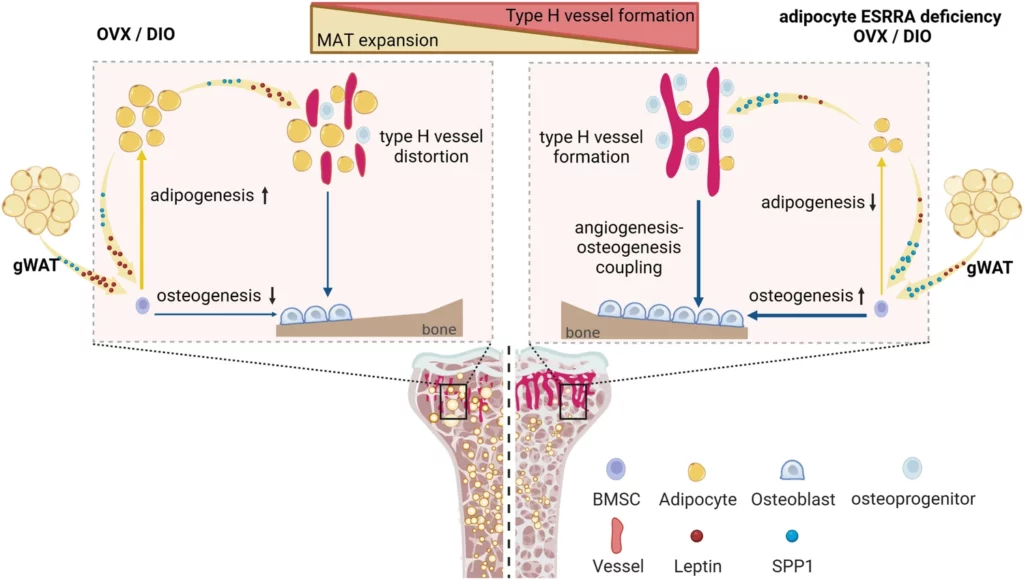
Targeting adipocyte ESRRA promotes osteogenesis and vascular formation in adipocyte-rich bone marrow
Tongling Huang, Zhaocheng Lu, Zihui Wang, Lixin Cheng, Lu Gao, Jun Gao, Ning Zhang, Chang-An Geng, Xiaoli Zhao, Huaiyu Wang, Chi-Wai Wong, Kelvin W. K. Yeung, Haobo Pan, William Weijia Lu, Min Guan
Excessive bone marrow adipocytes (BMAds) accumulation often occurs under diverse pathophysiological conditions associated with bone deterioration. Estrogen-related receptor α (ESRRA) is a key regulator responding to metabolic stress. Here, we show that adipocyte-specific ESRRA deficiency preserves osteogenesis and vascular formation in adipocyte-rich bone marrow upon estrogen deficiency or obesity. Mechanistically, adipocyte ESRRA interferes with E2/ESR1 signaling resulting in transcriptional repression of secreted phosphoprotein 1 (Spp1); yet positively modulates leptin expression by binding to its promoter. ESRRA abrogation results in enhanced SPP1 and decreased leptin secretion from both visceral adipocytes and BMAds, concertedly dictating bone marrow stromal stem cell fate commitment and restoring type H vessel formation, constituting a feed-forward loop for bone formation. Pharmacological inhibition of ESRRA protects obese mice against bone loss and high marrow adiposity. Thus, our findings highlight a therapeutic approach via targeting adipocyte ESRRA to preserve bone formation especially in detrimental adipocyte-rich bone milieu.