Bone marrow adipogenic lineage precursors are the major regulator of bone resorption in adult mice
Jiawei Lu1,2, Qi He1, Huan Wang1, Lutian Yao1, Michael Duffy1, Hanli Guo1, Corben Braun1, Yilu Zhou1, Qiushi Liang1, Yuewei Lin1, Shovik Bandyopadhyay3,4, Kai Tan3,4, Yongwen Choi5, X Sherry Liu1, Ling Qin1.
- 1Department of Orthopaedic Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA.
- 2Department of Spine Surgery, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, 200092, China.
- 3Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA.
- 4Center for Childhood Cancer Research, The Children’s Hospital of Philadelphia, Philadelphia, PA, 19104, USA.
- 5Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA.
- 6Department of Orthopaedic Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA.
Correspondence:Ling Qin: qinling@pennmedicine.upenn.edu
Bone Research 2025 Mar 19;13(1):39.
PMID: 40102423 | PMCID: PMC11920254 | DOI: 10.1038/s41413-025-00405-4
Key Findings
In this study the authors demonstrate that Marrow Adipogenic Lineage Progenitors (MALPs), and not osteocytes, are the dominant source of the pro-osteoclastic protein RANKL in adult mouse bone marrow. Depleting RANKL in MALPs led to a rapid increase in trabecular bone mass. Finally, the authors also showed that MALP-derived RANKL drives ovariectomy-induced bone loss. Altogether, this establishes a crucial role for the precursor cells of bone marrow adipose tissue in facilitating bone remodeling.
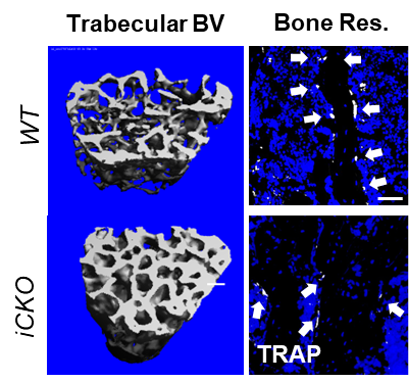
Figure: Trabecular bone volume is increased and bone resorption is decreased 6-weeks after RANKL deletion from MALPs.
Clinical implications of bone marrow adiposity identified by phenome-wide association and Mendelian randomization in the UK Biobank
- 1 Centre for Global Health, Usher Institute, University of Edinburgh, Edinburgh, UK.
- 2 Institute for Neuroscience and Cardiovascular Research, The University of Edinburgh, Edinburgh, UK.
- 3 Edinburgh Imaging, University of Edinburgh, The Queen’s Medical Research Institute, Edinburgh BioQuarter, 47 Little France Crescent, Edinburgh, UK.
- 4 School of Mathematics and Computer Sciences, Heriot-Watt University, Edinburgh, UK.
- 5 Archimedes Unit, Athena Research Centre, Artemidos 1, Marousi, Greece.
- 6 Univ. Lille, CHU Lille, Marrow Adiposity and Bone Laboratory (MABlab) ULR 4490, Department of Rheumatology, Lille, France.
- 7 Department of Big Data in Health Science, School of Public Health and The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.
- 8 Medical Research Council Human Genetics Unit, Medical Research Council Institute of Genetics & Molecular Medicine, University of Edinburgh, Edinburgh, UK.
- 9 Danish Institute for Advanced Study (DIAS), Epidemiology, Biostatistics and Biodemography, Department of Public Health, University of Southern Denmark, Odense, Denmark.
- 10 Centre for Clinical Brain Sciences, University of Edinburgh, The Queen’s Medical Research Institute, Edinburgh BioQuarter, 47 Little France Crescent, Edinburgh, UK.
- 11 Centre for Global Health, Usher Institute, University of Edinburgh, Edinburgh, UK. E.Theodoratou@ed.ac.uk.
- 12 Edinburgh Cancer Research Centre, Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK. E.Theodoratou@ed.ac.uk.
- 13 Institute for Neuroscience and Cardiovascular Research, The University of Edinburgh, Edinburgh, UK. W.Cawthorn@ed.ac.uk.
Correspondence:Evropi Theodoratou: E.Theodoratou@ed.ac.uk; William P Cawthorn:W.Cawthorn@ed.ac.uk
Nature Communications 2025 Sep 23;16(1):8332.
PMID: 40987763 | PMCID: PMC12457654 |DOI: 10.1038/s41467-025-63395-1
Key findings (Description Up to 150 words): The article “Clinical implications of bone marrow adiposity identified by phenome-wide association and Mendelian randomization in the UK Biobank” presents comprehensive research on the role of bone marrow adipose tissue (BMAT) in human health. Bone marrow fat fraction was measured using MRI in over 48,000 UK Biobank participants at multiple skeletal sites. Phenome-wide association studies (PheWAS) revealed links between marrow adiposity and 47 diseases across 12 categories, including osteoporosis, fractures, type 2 diabetes, cardiovascular diseases, and cancers. Interestingly, type 2 diabetes showed positive association with spinal marrow adiposity but negative with femoral marrow adiposity. These findings suggest marrow adiposity could serve as a biomarker or therapeutic target for several diseases. These findings highlight the critical importance of analyzing BMFF at multiple anatomical locations and underscore the concept that the pathophysiological functions of BMAT are skeletal site dependent. |
Skeletal Site-Specific Lipid Profile and Hematopoietic Progenitors of Bone Marrow Adipose Tissue in Patients Undergoing Primary Hip Arthroplasty
- 1 Group for Hematology and Stem Cells, Institute for Medical Research, National Institute of Republic of Serbia, University of Belgrade, 11000 Belgrade, Serbia.
- 2 Institute for Orthopedy Banjica, 11000 Belgrade, Serbia.
- 3 Faculty of Medicine, University of Belgrade, 11000 Belgrade, Serbia.
- 4 Centre of Research Excellence in Nutrition and Metabolism, Institute for Medical Research, National Institute of Republic of Serbia, University of Belgrade, 11000 Belgrade, Serbia.
- 5 Faculty of Mathematics, University of Belgrade, 11000 Belgrade, Serbia.
- 6 Institute for Artificial Intelligence Research and Development of Serbia, 21000 Novi Sad, Serbia.
Correspondence:Drenka Trivanović, drenka.trivanovic@imi.bg.ac.rs
Metabolites 2025 Jan 4;15(1):16.
PMID: 39852359 | PMCID: PMC11767117 | DOI: 10.3390/metabo15010016
Abstract
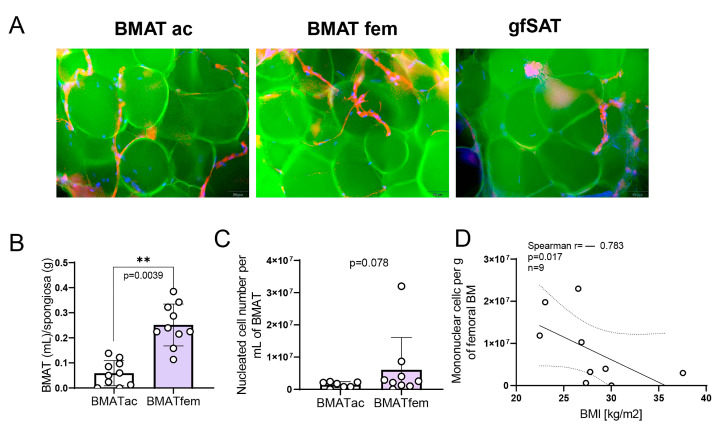
Methods: Acetabular and femoral bone marrow (BM) and gluteofemoral subcutaneous adipose tissue (gfSAT) were obtained from matched patients undergoing hip replacement surgery. BM, BMAT, and gfSAT were explored at the levels of total lipids, fatty acids, and cells by using thin-layer and gas chromatography, ex vivo cellular assays, and flow cytometry.
Results: BMAT content was significantly higher in femoral than in acetabular BM. Total lipid analyses revealed significantly lower triglyceride content in femoral than in acetabular BMAT and gfSAT. Frequencies of saturated palmitic, myristic, and stearic acids were higher in femoral than in acetabular BMAT and gfSAT. The content of CD45+CD34+ cells within femoral BMAT was higher than in acetabular BMAT or gfSAT. This was associated with a higher incidence of total clonogenic hematopoietic progenitors and late erythroid colonies CFU-E in femoral BMAT when compared to acetabular BMAT, similar to their BM counterparts.
Conclusions: Collectively, our results indicate that the lipid profiles of hip bone and femoral BMAT impose significantly different microenvironments and distributions of cells with hematopoietic potential. These findings might bring forth new inputs for defining BMAT biology and setting novel directions in OA disease investigations.
Keywords: bone marrow adipose tissue; fatty acid; hematopoietic progenitors; osteoarthritis; stem cells.
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Implication of bone marrow adipose tissue in bone homeostasis during osteoarthritis
- 1Sorbonne Université, INSERM, Centre de Recherche Saint-Antoine, CRSA, F-75012 Paris, France.
- 2Sorbonne Université, INSERM, Centre de Recherche Saint-Antoine, CRSA, F-75012 Paris, France; Sorbonne Université, CNRS, Laboratoire de
Réactivité de Surface, LRS, F-75005, Paris, France. - 3Hemato-Oncology Program. CIMA Universidad de Navarra-IdiSNA, Pamplona, Spain.
- 4Marrow Adiposity and Bone Lab (MABLab) ULR4490, Université du Littoral Côte d’Opale, F-62200 Boulogne sur Mer, Univ. Lille, F-59000 Lille, CHU Lille, F-59000 Lille, France.
- 5Hemato-Oncology Program. CIMA Universidad de Navarra-IdiSNA, Pamplona, Spain; Computational Biology Program, CIMA Universidad de
Navarra-IdiSNA, Pamplona, Spain. - 6Sorbonne Université, CNRS, Laboratoire de Réactivité de Surface, LRS, F-75005, Paris, France.
- 7Clinique Maussins-Nollet, F-75019 Paris, France.
- 8Hemato-Oncology Program. CIMA Universidad de Navarra-IdiSNA, Pamplona, Spain; Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain; Hematology and Cell Therapy Department, Clinica Universidad de Navarra, IdiSNA, Pamplona, Spain; Cancer Center
Universidad de Navarra (CCUN), Pamplona, Spain. - 9Sorbonne Université, INSERM, Centre de Recherche Saint-Antoine, CRSA,F-75012 Paris, France; Rheumatology Department, AP-HP Saint-AntoineHospital, 184, Rue du Faubourg Saint-Antoine, 75012 Paris, France.
- 10Hemato-Oncology Program. CIMA Universidad de Navarra-IdiSNA, Pamplona, Spain; Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain; Cancer Center Universidad de Navarra (CCUN), Pamplona, Spain.
- 11 Université de Lyon – Université Jean Monnet, INSERM U1059, Faculté de Médecine, F-42270 Saint-Priest en Jarez, France.
- 12Sorbonne Université, INSERM, Centre de Recherche Saint-Antoine, CRSA,
F-75012 Paris, France. Electronic address:
Correspondence: Xavier Houard, xavier.houard@sorbonne-universite.fr.
- PMID: 40154729 | DOI: 10.1016/j.joca.2025.03.004
Abstract
Objective: To explore the role of bone marrow adipocytes (BMAds) in osteoarthritis (OA).
Methods: Male and female C57BL/6 mice (n=4/group) underwent meniscectomy (MNX) or SHAM surgery. OA was determined using Osteoarthritis Research Society International (OARSI) score, and the number of perilipin+ adipocytes was quantified. Mesenchymal Stromal Cells (MSCs) from MNX and SHAM mice were differentiated into osteoblasts and adipocytes. Human adipocytes and MSCs (n=8) were enzymatically isolated from epiphyseal and metaphyseal marrow, and from subcutaneous adipose tissue (SCAT) of hip OA patients. Human OA MSCs were differentiated into osteoblasts and adipocytes (OA-Diff-hAdipo). Gene expression patterns of epiphyseal and metaphyseal BMAds, SCAT adipocytes and OA-Diff-hAdipo were evaluated by RNAseq (n=4). The effect conditioned media from OA epiphyseal bone (n=5) on the alkaline phosphatase (ALP) activity and mineralization kinetics was assessed in vitro.
Results: Increase in BMAd density was positively correlated with cartilage degradation in MNX mice. OA modified the differentiation capacity of MSCs, accelerating adipocyte differentiation and failing to produce osteoblasts in both human and mice. Human epiphyseal, metaphyseal and SCAT adipocytes from the same OA patients each displayed a specific transcriptome, suggesting different functions. Enrichment analysis defined metaphyseal OA-BMAds as cells implicated in hematopoietic stem cell differentiation. On the other hand, epiphyseal OA-BMAds were considered as osteogenic cells showing an up-regulation of genes related to bone mineralization and remodeling. Specifically, OA epiphysis-secreted molecules decreased ALP activity and altered in vitro the mineralization process.
Conclusion: All these results support the emergence of BMAds as new cell partners in OA, opening new venues for therapeutic approaches.
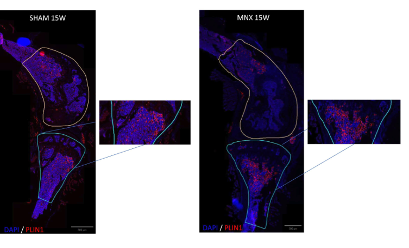
Figure 1C. Cartilage degradation is positively correlated to bone marrow adiposity in the double MNX mouse model. Perilipin (Plin1) immunofluorescence of SHAM (Scale bar: 800 μm) and MNX (Scale bar: 500 μm) mice at 15 W post-surgery, showing an increased presence of adipocytes in the marrow of the epiphysis and metaphysis of MNX mice. Areas of quantification of Plin1+ cells in the tibiae and femur are highlighted in blue and yellow respectively. Plin1+ cells are shown in red, DAPI in blue. Zoom areas are shown for the tibiae of MNX and SHAM mice showing Plin1+ cells.
Keywords: Bone marrow adipose tissue; Epiphysis; Mineralization; Osteoarthritis; Subchondral bone.
Copyright © 2025 The Authors. Published by Elsevier Ltd. All rights reserved.
Superstable lipid vacuoles endow cartilage with its shape and biomechanics
Raul Ramos1,2, Kim T Pham1,2, Richard C Prince3,4, Leith B Leiser-Miller5, Maneeshi S Prasad6,7, Xiaojie Wang1,2, Rachel C Nordberg4, Benjamin J Bielajew4, Jerry C Hu4, Kosuke Yamaga1,2, Ji Won Oh1,2,8,9,10, Tao Peng11,12, Rupsa Datta4, Aksana Astrowskaja13, Axel A Almet11,14, John T Burns1, 2, Yuchen Liu1,2, Christian Fernando Guerrero-Juarez1,2,11,12, Bryant Q Tran1,2 , Yi-Lin Chu1,2, Anh M Nguyen1,2, Tsai-Ching Hsi1,2, Norman T-L Lim15, Sandra Schoeniger16,17, Ruiqi Liu1,2, Yun-Ling Pai2,18, Chella K Vadivel2,19, Sandy Ingleby20 , Andrew E McKechnie21,22, Frank van Breukelen23, Kyle L Hoehn24, John J Rasweiler 4th25, Michinori Kohara26 , William J Loughry27, Scott H Weldy28, Raymond Cosper29, Chao-Chun Yang30,31 , Sung-Jan Lin18, Kimberly L Cooper32, Sharlene E Santana5,33, Jeffrey E Bradley33, Michael A Kiebish3, Michelle Digman12,35, David E James36, Amy E Merrill37, Qing Nie11,12,14, Thomas F Schilling1,12,14, Aliaksandr A Astrowski38, Eric O Potma3, Martín I García-Castro6, Kyriacos A Athanasiou4, Richard R Behringer39, Maksim V Plikus1,2,12,14
Affiliations: 1Department of Developmental and Cell Biology, University of California, Irvine, Irvine, CA, USA.2Sue and Bill Gross Stem Cell Research Center, University of California, Irvine, Irvine, CA, USA. 3Department of Chemistry, University of California, Irvine, Irvine, CA, USA.4 Department of Biomedical Engineering, University of California, Irvine, Irvine, CA, USA.5Department of Biology, University of Washington, Seattle, WA, USA. 6Division of Biomedical Sciences, School of Medicine, University of California, Riverside, Riverside, CA, USA.7Department of Biochemistry, Jacobs School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY, USA.8Department of Anatomy, College of Medicine, Yonsei University, Seoul, Republic of Korea. 9Department of Anatomy, School of Medicine, Kyungpook National University, Daegu, Republic of Korea. 10Biomedical Research Institute, Kyungpook National University Hospital, Daegu, Republic of Korea.11Department of Mathematics, University of California, Irvine, Irvine, CA, USA. 12Center for Complex Biological Systems, University of California, Irvine, Irvine, CA, USA. 13Scientific Research Laboratory of Molecular Medicine, Grodna State Medical University, Grodna, Belarus.14NSF-Simons Center for Multiscale Cell Fate Research, University of California, Irvine, Irvine, CA, USA. 15National Institute of Education, Singapore, Republic of Singapore.16Institute of Veterinary Pathology, Leipzig University, Leipzig, Germany. 17Discovery Life Sciences Biomarker Services GmbH, Kassel, Germany. 18Institute of Biomedical Engineering, College of Medicine and College of Engineering, National Taiwan University, Taipei, Taiwan. 19LEO Foundation Skin Immunology Research Center, Department of Immunology and Microbiology, University of Copenhagen, Copenhagen, Denmark. 20Australian Museum, Sydney, NSW, Australia. 21Mammal Research Institute, Department of Zoology and Entomology, University of Pretoria, Hatfield, South Africa. 22South African National Biodiversity Institute, Pretoria, South Africa. 23School of Life Sciences, University of Nevada, Las Vegas, Las Vegas, NV, USA. 24School of Biotechnology and Biomolecular Sciences, University of New South Wales, Sydney, NSW, Australia. 25Department of Obstetrics and Gynecology, SUNY Downstate Medical Center, New York, NY, USA. 26Department of Microbiology and Cell Biology, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan. 27Valdosta State University, Valdosta, GA, USA. 28Serrano Animal and Bird Hospital, Lake Forest, CA, USA. 29Santa Ana Zoo, Santa Ana, CA, USA. 30Department of Dermatology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan. 31International Center for Wound Repair and Regeneration, National Cheng Kung University, Tainan, Taiwan. 32Department of Cell and Developmental Biology, University of California, San Diego, La Jolla, CA, USA. 33Department of Mammalogy, Burke Museum, University of Washington, Seattle, WA, USA. 34BPGbio, Inc., Framingham, MA, USA. 35Department of Chemical Engineering and Materials Science, University of California, Irvine, Irvine, CA, USA. 36Charles Perkins Centre, School of Life and Environmental Sciences and School of Medical Sciences, University of Sydney, Sydney, NSW, Australia.37Center for Craniofacial Molecular Biology, Ostrow School of Dentistry, University of Southern California, Los Angeles, CA, USA. 38The Institute of Biochemistry of Biologically Active Compounds, Grodna, Belarus. 39Department of Genetics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Correspondence: Maksim V Plikus, plikus@uci.edu
Abstract
Conventionally, the size, shape, and biomechanics of cartilages are determined by their voluminous extracellular matrix. By contrast, we found that multiple murine cartilages consist of lipid-filled cells called lipochondrocytes. Despite resembling adipocytes, lipochondrocytes were molecularly distinct and produced lipids exclusively through de novo lipogenesis. Consequently, lipochondrocytes grew uniform lipid droplets that resisted systemic lipid surges and did not enlarge upon obesity. Lipochondrocytes also lacked lipid mobilization factors, which enabled exceptional vacuole stability and protected cartilage from shrinking upon starvation. Lipid droplets modulated lipocartilage biomechanics by decreasing the tissue’s stiffness, strength, and resilience. Lipochondrocytes were found in multiple mammals, including humans, but not in nonmammalian tetrapods. Thus, analogous to bubble wrap, superstable lipid vacuoles confer skeletal tissue with cartilage-like properties without “packing foam–like” extracellular matrix.
Graphical Abstract
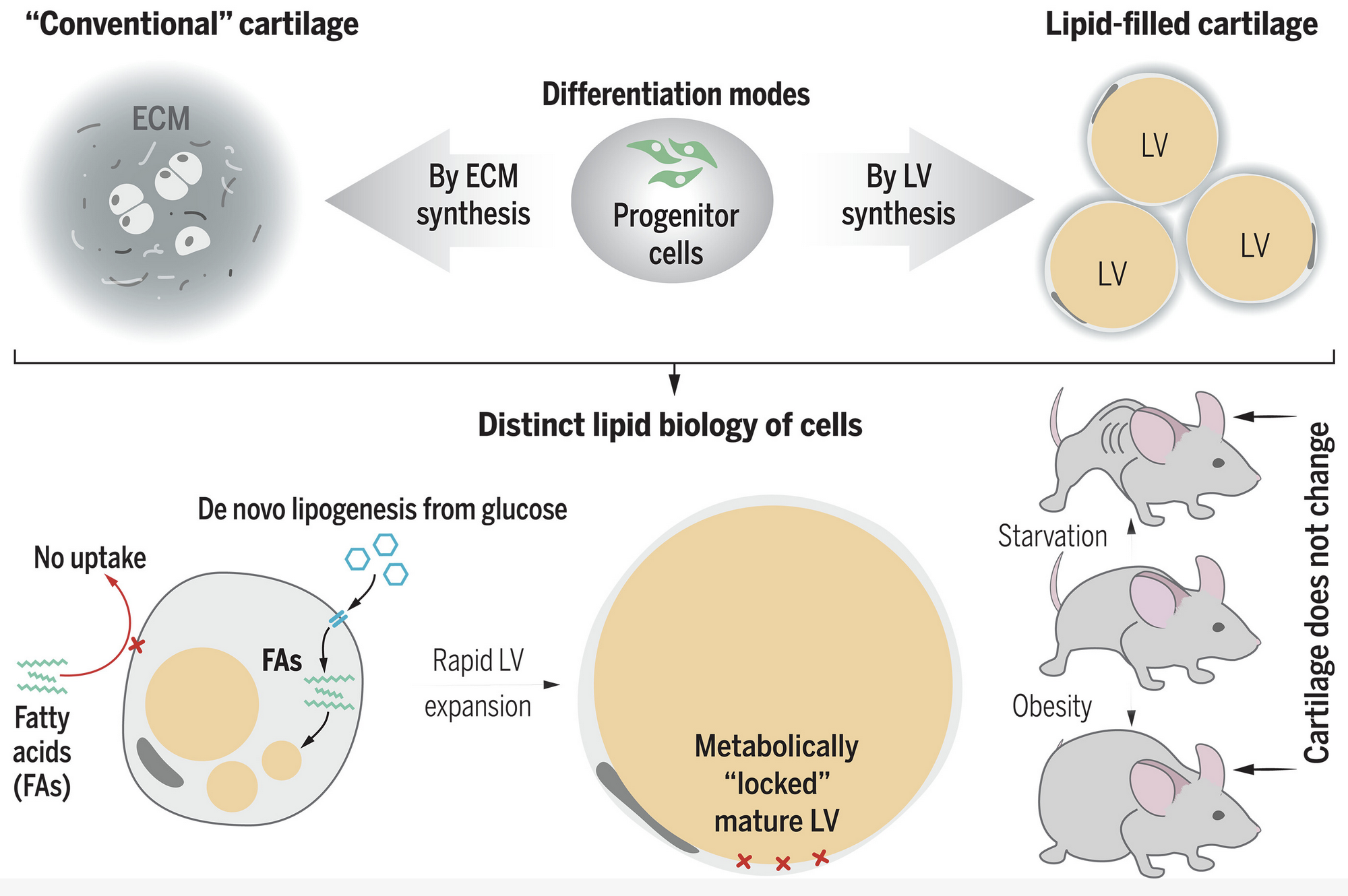
Lipid-filled cartilage of mammals. Unlike in conventional cartilage, the form and function of lipid-filled cartilage derives from giant lipid vacuoles (center). Vacuolated cartilage in mammals represents convergent evolution with the notochord, which has cells containing giant aqueous vacuoles. Developing cartilage grows vacuoles by a tightly controlled biochemical pathway (bottom). Mature lipocartilage maintains stable vacuoles by turning off lipid mobilization. This unusual molecular biology safeguards the vacuoles from unintended size fluctuations upon systemic metabolic disturbances. ECM, extracellular matrix.
Copyright © 2025 the authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original US government works.
Uncovering mechanisms of thiazolidinediones on osteogenesis and adipogenesis using spatial fluxomics
Kristyna Brejchova1, Michal Rahm2, Andrea Benova3, Veronika Domanska1, Paul Reyes-Gutierez2, Martina Dzubanova4, Radka Trubacova1, Michaela Vondrackova1, Tomas Cajka5, Michaela Tencerova3, Milan Vrabel2, Ondrej Kuda6
1 Laboratory of Metabolism of Bioactive Lipids, Institute of Physiology of the Czech Academy of Sciences, Videnska 1083, 14200 Prague, Czechia.
2 Chemistry of Bioconjugates, Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Flemingovo nám. 2, 166 10 Prague, Czechia.
3 Laboratory of Molecular Physiology of Bone, Institute of Physiology of the Czech Academy of Sciences, Videnska 1083, 14200 Prague, Czechia.
4 Laboratory of Molecular Physiology of Bone, Institute of Physiology of the Czech Academy of Sciences, Videnska 1083, 14200 Prague, Czechia; Faculty of Science, Charles University, Albertov 6, 128 00 Prague, Czech Republic.
5 Laboratory of Translational Metabolism, Institute of Physiology of the Czech Academy of Sciences, Videnska 1083, 14200 Prague, Czechia.
6 Laboratory of Metabolism of Bioactive Lipids, Institute of Physiology of the Czech Academy of Sciences, Videnska 1083, 14200 Prague, Czechia.
Correspondence: ondrej.kuda@fgu.cas.cz.
Abstract
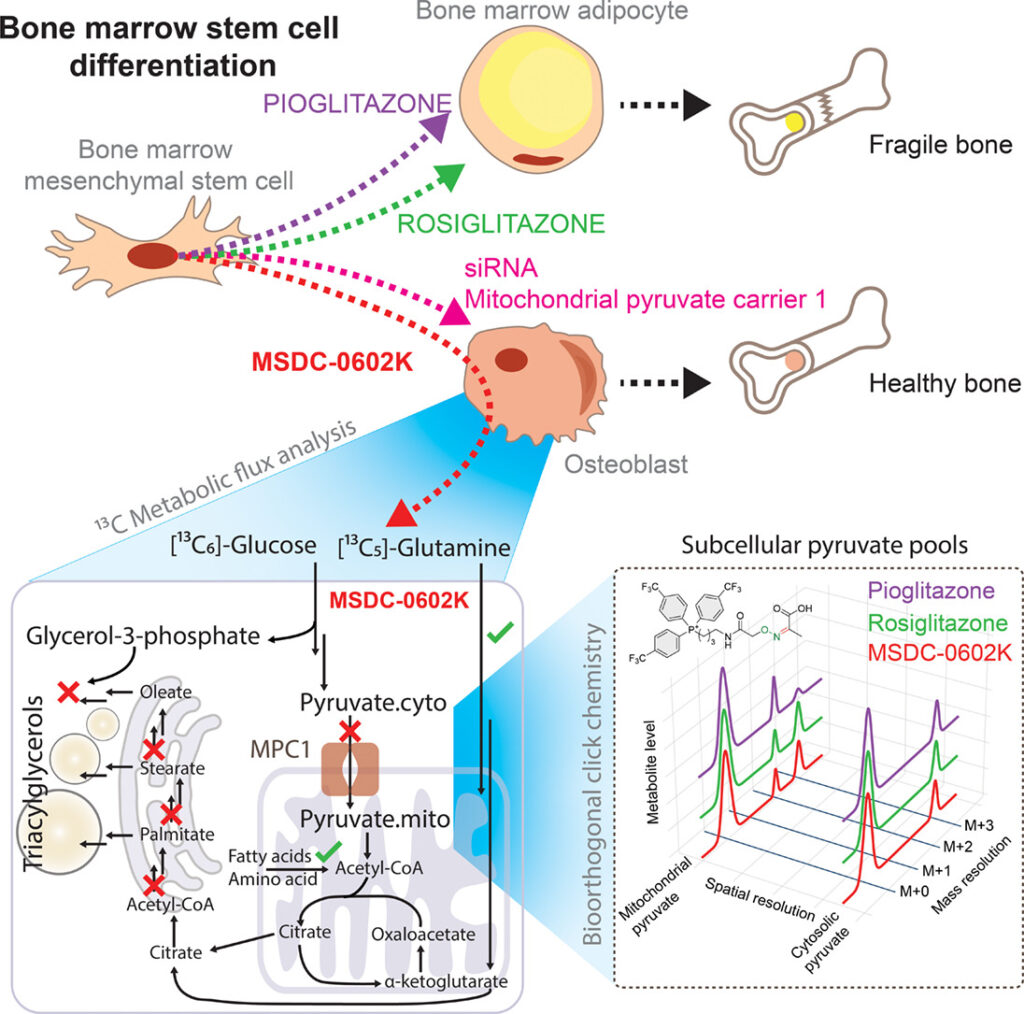
Objective: Insulin-sensitizing drugs, despite their broad use against type 2 diabetes, can adversely affect bone health, and the mechanisms underlying these side effects remain largely unclear. Here, we investigated the different metabolic effects of a series of thiazolidinediones, including rosiglitazone, pioglitazone, and the second-generation compound MSDC-0602K, on human mesenchymal stem cells (MSCs).
Methods: We developed 13C subcellular metabolomic tracer analysis measuring separate mitochondrial and cytosolic metabolite pools, lipidomic network-based isotopologue models, and bioorthogonal click chemistry, to demonstrate that MSDC-0602K differentially affected bone marrow-derived MSCs (BM-MSCs) and adipose tissue-derived MSCs (AT-MSCs). In BM-MSCs, MSDC-0602K promoted osteoblastic differentiation and suppressed adipogenesis. This effect was clearly distinct from that of the earlier drugs and that on AT-MSCs.
Results: Fluxomic data reveal unexpected differences between this drug’s effect on MSCs and provide mechanistic insight into the pharmacologic inhibition of mitochondrial pyruvate carrier 1 (MPC). Our study demonstrates that MSDC-0602K retains the capacity to inhibit MPC, akin to rosiglitazone but unlike pioglitazone, enabling the utilization of alternative metabolic pathways. Notably, MSDC-0602K exhibits a limited lipogenic potential compared to both rosiglitazone and pioglitazone, each of which employs a distinct lipogenic strategy.
Conclusions: These findings indicate that the new-generation drugs do not compromise bone structure, offering a safer alternative for treating insulin resistance. Moreover, these results highlight the ability of cell compartment-specific metabolite labeling by click reactions and tracer metabolomics analysis of complex lipids to discover molecular mechanisms within the intersection of carbohydrate and lipid metabolism.
Graphical Abstract
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
KIAA1199 (CEMIP) regulates adipogenesis and whole-body energy metabolism
- 1Guangxi Key Laboratory of Tumor Immunology and Microenvironment Regulation, Guilin Medical University, Guilin, Guangxi, 541199, China.
- 2 Department of Endocrinology and Metabolism, Endocrine Research Laboratory (KMEB), Odense University Hospital & University of Southern Denmark, Odense, Dk-5230, Denmark.
- 3 Department of Endocrinology and Metabolism, Endocrine Research Laboratory (KMEB), Odense University Hospital & University of Southern Denmark, Odense, Dk-5230, Denmark.
- 4 Guangxi Key Laboratory of Tumor Immunology and Microenvironment Regulation, Guilin Medical University, Guilin, Guangxi, 541199, China.
- 5 Department of Pathophysiology, Centre for Research on Diabetes, Metabolism and Nutrition, Third Faculty of Medicine, Charles University, Prague, Czech Republic.
- 6 Biomechanics Lab, Institute of Mechanics, Materials, and Civil Engineering, KU Leuven, Leuven, Belgium.
- 7 Biomechanics Section, Department of Mechanical Engineering, KU Leuven, Leuven, Belgium.
- 8 Institute of Pathology, University of Southern Denmark, Odense, Denmark.
- 9 Department of Medicine (H7), Karolinska Institute, Karolinska University Hospital, Stockholm, Sweden.
- 10 Department of Endocrinology and Metabolism, Endocrine Research Laboratory (KMEB), Odense University Hospital & University of Southern Denmark, Odense, Dk-5230, Denmark.
- 11 Khalifa University, College of Medicine and Health Sciences & Biotechnology Center (BTC), Abu Dhabi, United Arab Emirates.
ABSTRACT
An increasing number of studies have characterized the bone as an endocrine organ, and that bone secreted factors may not only regulate local bone remodeling, but also other tissues and whole-body metabolic functions. The precise nature of these regulatory factors and their roles at bridging the bone, bone marrow adipose tissue, extramedullary body fat and whole-body energy homeostasis are being explored. In this study, we report that KIAA1199, a secreted factor produced from bone and bone marrow, previously described as an inhibitor of bone formation, also plays a role at promoting adipogenesis. KIAA1199-deficient mice exhibit reduced bone marrow adipose tissue, subcutaneous and visceral fat tissue mass, blood cholesterol, triglycerides, free fatty acids and glycerol, as well as improved insulin sensitivity in skeletal muscle, liver and fat. Moreover, these mice are protected from the detrimental effects of high-fat diet feeding, with decreased obesity, lower blood glucose and glucose tolerance, as well as decreased adipose tissue inflammation, insulin resistance and hepatic steatosis. In human studies, plasma levels of KIAA1199 or its expression levels in adipose tissue are positively correlated with insulin resistance and blood levels of cholesterol, triglycerides, free fatty acids, glycerol, fasting glucose and HOMA-IR. Mechanistically, KIAA1199 mediates its effects on adipogenesis through modulating osteopontin-integrin and AKT / ERK signaling. These findings provide evidence for the role of bone secreted factors on coupling bone, fat and whole-body energy homeostasis.
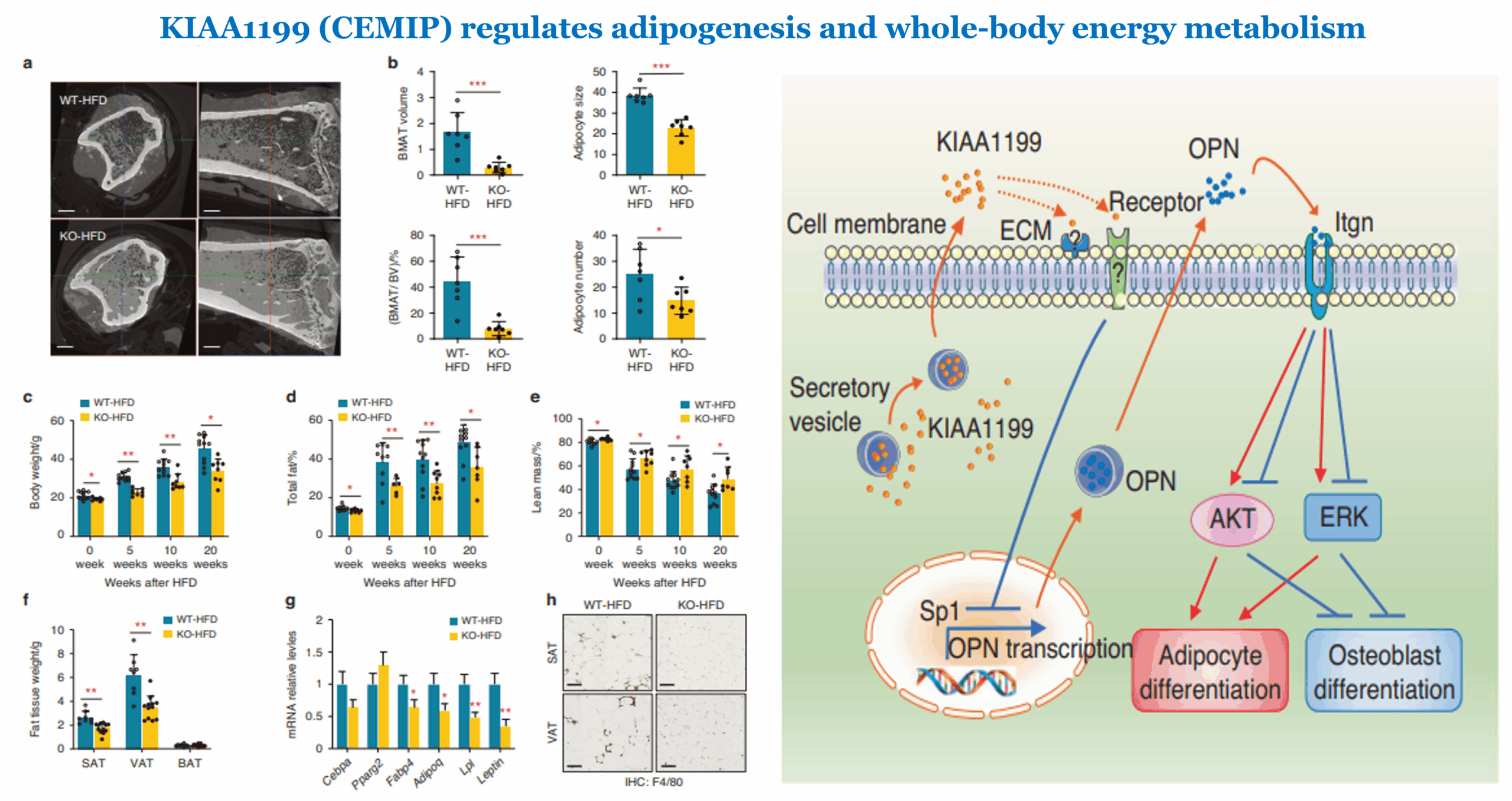
Left(Figure 4): KIAA1199 knockout (KO) mice are protected from obesity following high-fat-diet (HFD) feeding. KIAA1199 KO and WT female mice received 20 weeks high fat diet (HFD). a Hf-POM-based CE-µCT scan of the tibiae show bone marrow adipocytes tissue (BMAT) as black spots, scale bar= 5 μm. b Quantitation of BMAT volume (mm3), BMAT volume ratio of bone marrow (%, V/V), BMAT number (×103/mm3) and size (µm3 × 103/mm3) were analyzed, n = 7.
Right: (Figure 7h): A schematic diagram of the possible mechanisms of KIAA1199 interacts with OPN/integrin/AKT/ERK axis on regulating adipogenesis and osteogenesis.
© 2025. The Author(s).
Subchondral bone marrow adipose tissue lipolysis regulates bone formation in hand osteoarthritis
- Plastic and Hand Surgery, Department of Musculoskeletal Medicine, University Hospital Lausanne and University of Lausanne (CHUV-UNIL), Lausanne, Switzerland; Department of Hand Surgery, Balgrist University Clinic, Zürich, Switzerland.
- 2 Rheumatology, Department of Musculoskeletal Medicine, University Hospital Lausanne and University of Lausanne (CHUV-UNIL), Lausanne, Switzerland.
- 3 Orthopaedics, Department of Musculoskeletal Medicine, University Hospital Lausanne and University of Lausanne (CHUV-UNIL), Lausanne, Switzerland.
- 4 Rheumatology, Department of Musculoskeletal Medicine, University Hospital Lausanne and University of Lausanne (CHUV-UNIL), Lausanne, Switzerland. Electronic address: Jeroen.Geurts@chuv.ch.
ABSTRACT
Objective: Bone marrow adipose tissue (BMAT) is emerging as an important regulator of bone formation and energy metabolism. Lipolysis of BMAT releases glycerol and fatty acid substrates that are catabolized by osteoblasts. Here, we investigated whether BMAT lipolysis is involved in subchondral bone formation in hand osteoarthritis (OA).
Methods: Subchondral BMAT lipolysis and bone marrow adipocyte (BMAd) morphology were studied in clinical specimens of carpometacarpal (CMC-1) and distal interphalangeal joint OA. BMAd size, osteoblast numbers and expression of lipolysis enzymes (ATGL, phospho-HSL, MGLL) were compared between regions of low and high bone formation. Free fatty acids, glycerol and bone biomarkers were measured in osteochondral explants.
Results: Subchondral BMAd size was positively correlated with BMI (r = 0.60, [0.082,0.87]) and reduced in regions of high bone formation (-1149 µm2, [-1977,-726.2]). Osteoblast numbers were negatively correlated with BMAd size (r = -0.48, [-0.73,-0.12]). All lipolysis enzymes were expressed in both in BMAds and activated osteoblasts and the area percentages of ATGL (+2.26% [0.19,3.47]), phospho-HSL (+1.57% [0.31,6.48]) and MGLL (+4.04% [1.09,5.69]) were increased in regions of high bone formation. Secreted glycerol levels, but not free fatty acids, were correlated with bone formation markers pro-collagen type I (rho = 0.90) and alkaline phosphatase (rho = 0.78).
Conclusion: Our findings reveal a previously unrecognized role of BMAT lipolysis in regulating bone formation in hand OA, which may be modulated by BMI.
Keywords: Bone; Bone marrow adipose tissue; Hand OA; Lipolysis; Osteoblast.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figure 2. Reduction of BMAd size in areas of high remodeling.

Fig. 2: Typical morphology of subchondral bone and BMAT in low and high remodeling areas visualized by whole-mount Oil red-O and Hoechst staining. Scale bar = 200 μm. (A) Typical morphology of subchondral BMAT in areas of low and high remodeling visualized by Safranin-O staining. Osteoblasts indicated by red arrowheads. Scale bars = 500 μm (low magnification) and 100 μm (high magnification). (B) Median BMAd size quantified from high magnification images. P-value < 0.0005 by Wilcoxon matched-pairs signed rank test, (n = 13) (C) Osteoblast numbers quantified from high magnification images. P-value < 0.001 by paired t-test, (n = 13) (D). Scatterplot showing correlation between median BMAd size and osteoblast numbers in low and high remodeling areas of thirteen CMC-1 joints and the regression line with 95% confidence intervals. (E).
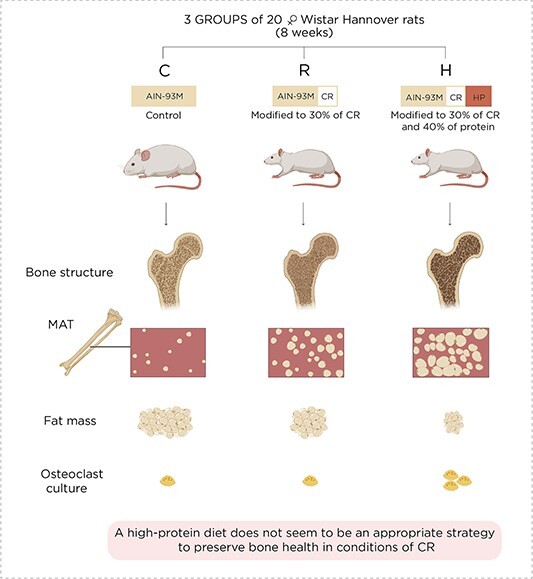
Low-calorie and high-protein diet has diverse impacts on the muscle, bone, and bone marrow adipose tissues
- 1 Department of Internal Medicine, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto 14.049-900, Brazil.
- 2 Department of Bio-Molecular Sciences, School of Pharmaceutical Science, University of São Paulo, Ribeirão Preto 14.040-903, Brazil.
- 3 Department of Internal Medicine, School of Medicine, University of São Paulo, São Paulo 01.246-903, Brazil.
- 4 Division of Endocrinology, Federal University of Sergipe, Aracaju, Sergipe 49.060-108, Brazil
- PMID: 39677928
- PMCID: PMC11646085
- DOI: 10.1093/jbmrpl/ziae150
ABSTRACT
The present study was designed to evaluate the influence of a high-protein diet under conditions of calorie restriction (CR) in the muscle, adipose tissue, bone, and marrow adipose tissue (MAT). It included three groups of 20 female Wistar Hannover rats, fed with the following diets for 8 wk: control group (C) fed with an AIN93M diet, CR group (R) fed with an AIN-93M diet modified to 30% CR, and CR + high-protein group (H) fed with an AIN-93M diet modified to 30% CR with 40% protein. Body composition was determined by DXA. The femur was used for histomorphometry and the estimation of adipocytes. Microcomputed tomography (μCT) was employed to analyze the bone structure. Hematopoietic stem cells from the bone marrow were harvested for osteoclastogenesis. Body composition revealed that the gain in lean mass surpassed the increase in fat mass only in the H group. Bone histomorphometry and μCT showed that a high-protein diet did not mitigate CR-induced bone deterioration. In addition, the number of bone marrow adipocytes and the differentiation of hematopoietic stem cells into osteoclasts were higher in H than in the other groups. These results indicated that under CR, a high-protein diet was beneficial for muscle mass. However, as the μCT scanning detected significant bone deterioration, this combined diet might accentuate the detrimental effect on the skeleton caused by CR. Remarkably, the H group rats exhibited greater MAT expansion and elevated hematopoietic stem cell differentiation into osteoclasts than the CR and control counterparts. These data suggest that a high protein may not be an appropriate strategy to preserve bone health under CR conditions.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Figure 1. Graphical Abstract and Lay Summary
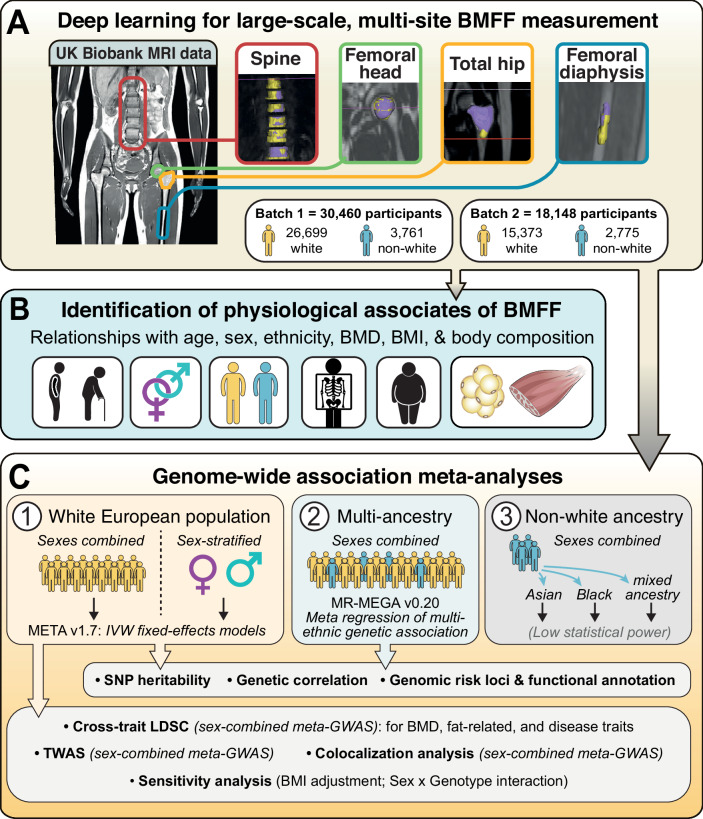
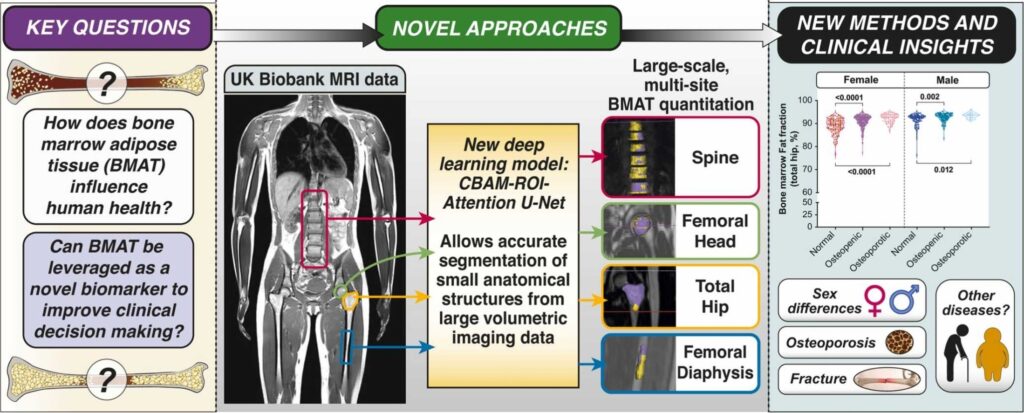
Deep learning and genome-wide association meta-analyses of bone marrow adiposity in the UK Biobank
- Centre for Global Health and Molecular Epidemiology, Usher Institute, University of Edinburgh, Edinburgh, UK.
- 2 University/BHF Centre for Cardiovascular Science, University of Edinburgh, The Queen’s Medical Research Institute, Edinburgh BioQuarter, 47 Little France Crescent, Edinburgh, UK.
- 3 Edinburgh Imaging, University of Edinburgh, The Queen’s Medical Research Institute, Edinburgh BioQuarter, 47 Little France Crescent, Edinburgh, UK.
- 4 School of Mathematics and Computer Sciences, Heriot-Watt University, Edinburgh, UK.
- 5 Archimedes Unit, Athena Research Centre, Marousi, Greece.
- 6 Univ. Lille, CHU Lille, Marrow Adiposity and Bone Laboratory (MABlab) ULR 4490, Department of Rheumatology, Lille, France.
- 7 Department of Big Data in Health Science, School of Public Health and The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.
- 8 Medical Research Council Human Genetics Unit, Medical Research Council Institute of Genetics & Molecular Medicine, University of Edinburgh, Edinburgh, UK.
- 9 Danish Institute for Advanced Study (DIAS), Epidemiology, Biostatistics and Biodemography, Department of Public Health, University of Southern Denmark, Odense, Denmark.
- 10 Cancer Research UK Edinburgh Centre, Medical Research Council Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK.
- 11 Colon Cancer Genetics Group, Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK.
- 12 Centre for Clinical Brain Sciences, University of Edinburgh, The Queen’s Medical Research Institute, Edinburgh BioQuarter, 47 Little France Crescent, Edinburgh, UK.
- 13 Centre for Global Health and Molecular Epidemiology, Usher Institute, University of Edinburgh, Edinburgh, UK. E.Theodoratou@ed.ac.uk.
- 14 Edinburgh Cancer Research Centre, Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK. E.Theodoratou@ed.ac.uk.
- 15 University/BHF Centre for Cardiovascular Science, University of Edinburgh, The Queen’s Medical Research Institute, Edinburgh BioQuarter, 47 Little France Crescent, Edinburgh, UK. W.Cawthorn@ed.ac.uk.
Nature Communications 16(1):99 January 2, 2025
- PMID: 39747859
- PMCID: PMC11697225
- DOI: 10.1038/s41467-024-55422-4
ABSTRACT
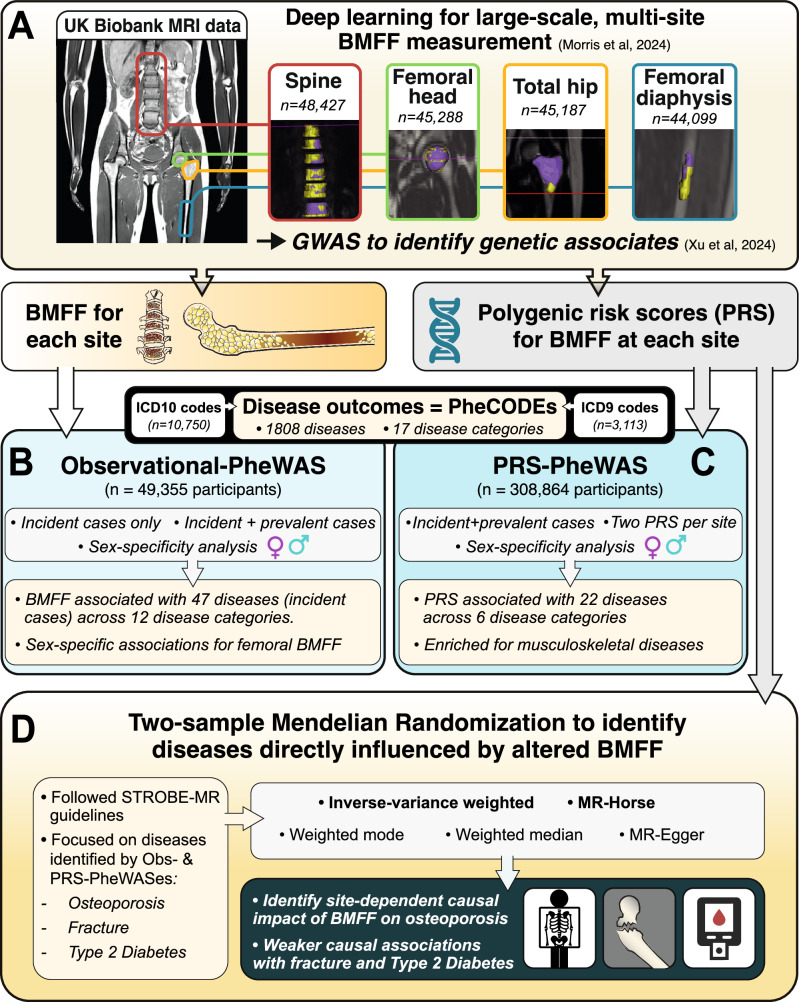
Bone marrow adipose tissue is a distinct adipose subtype comprising more than 10% of fat mass in healthy humans. However, the functions and pathophysiological correlates of this tissue are unclear, and its genetic determinants remain unknown. Here, we use deep learning to measure bone marrow adiposity in the femoral head, total hip, femoral diaphysis, and spine from MRI scans of approximately 47,000 UK Biobank participants, including over 41,000 white and over 6300 non-white participants. We then establish the heritability and genome-wide significant associations for bone marrow adiposity at each site. Our meta-GWAS in the white population finds 67, 147, 134, and 174 independent significant single nucleotide polymorphisms, which map to 54, 90, 43, and 100 genes for the femoral head, total hip, femoral diaphysis, and spine, respectively. Transcriptome-wide association studies, colocalization analyses, and sex-stratified meta-GWASes in the white participants further resolve functional and sex-specific genes associated with bone marrow adiposity at each site. Finally, we perform a multi-ancestry meta-GWAS to identify genes associated with bone marrow adiposity across the different bone regions and across ancestry groups. Our findings provide insights into BMAT formation and function and provide a basis to study the impact of BMAT on human health and disease.
Copyright © 2025 The Authors. Published by Springer-Nature. All rights reserved.
Figure 1. Study design.
Exploring contrast-enhancing staining agents for studying adipose tissue through contrast-enhanced computed tomography
Tim Balcaen1,2,3, Andrea Benova4,5, Flip de Jong6, Rodrigo de Oliveira Silva7, Tomas Cajka8, Dimitrios Sakellariou7, Michaela Tencerova4, Greet Kerckhofs2,3,9, Wim M De Borggraeve1
1 MolDesignS, Sustainable Chemistry for Metals and Molecules, Department of Chemistry, KU Leuven, Leuven, Belgium; Institute of Mechanics, Materials and Civil Engineering, Mechatronic, Electrical Energy and Dynamic Systems, UCLouvain, Louvain-la-Neuve, Belgium; Pole of Morphology, Institute of Experimental and Clinical Research, UCLouvain, Brussels, Belgium.
2 Laboratory of Molecular Physiology of Bone, Institute of Physiology of the Czech Academy of Sciences, Prague, Czech Republic; Faculty of Science, Charles University, Prague, Czech Republic.
3 Molecular Imaging and Photonics, Department of Chemistry, KU Leuven, Leuven, Belgium.
4 Membrane Separations, Adsorption, Catalysis, and Spectroscopy for Sustainable Solutions (cMACS), Department of Microbial and Molecular Systems, KU Leuven, Leuven, Belgium.
5 Laboratory of Translational Metabolism, Institute of Physiology of the Czech Academy of Sciences, Prague, Czech Republic.
6 Laboratory of Molecular Physiology of Bone, Institute of Physiology of the Czech Academy of Sciences, Prague, Czech Republic.
7 Institute of Mechanics, Materials and Civil Engineering, Mechatronic, Electrical Energy and Dynamic Systems, UCLouvain, Louvain-la-Neuve, Belgium; Pole of Morphology, Institute of Experimental and Clinical Research, UCLouvain, Brussels, Belgium; Department Materials Engineering, KU Leuven, Leuven, Belgium. Electronic address: greet.kerckhofs@uclouvain.be.
8 MolDesignS, Sustainable Chemistry for Metals and Molecules, Department of Chemistry, KU Leuven, Leuven, Belgium.
Journal of Lipid Research, Volume 65, Issue 7, July 2024, 100572
PMID: 38823780 | PMCID: PMC11259937 | DOI: 10.1016/j.jlr.2024.100572
ABSTRACT
Contrast-enhanced computed tomography offers a nondestructive approach to studying adipose tissue in 3D. Several contrast-enhancing staining agents (CESAs) have been explored, whereof osmium tetroxide (OsO4) is the most popular nowadays. However, due to the toxicity and volatility of the conventional OsO4, alternative CESAs with similar staining properties were desired. Hf-WD 1:2 POM and Hexabrix have proven effective for structural analysis of adipocytes using contrast-enhanced computed tomography but fail to provide chemical information. This study introduces isotonic Lugol’s iodine (IL) as an alternative CESA for adipose tissue analysis, comparing its staining potential with Hf-WD 1:2 POM and Hexabrix in murine caudal vertebrae and bovine muscle tissue strips. Single and sequential staining protocols were compared to assess the maximization of information extraction from each sample. The study investigated interactions, distribution, and reactivity of iodine species towards biomolecules using simplified model systems and assesses the potential of the CESA to provide chemical information. (Bio)chemical analyses on whole tissues revealed that differences in adipocyte gray values post-IL staining were associated with chemical distinctions between bovine muscle tissue and murine caudal vertebrae. More specific, a difference in the degree of unsaturation of fatty acids was identified as a likely contributor, though not the sole determinant of gray value differences. This research sheds light on the potential of IL as a CESA, offering both structural and chemical insights into adipose tissue composition.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Progression of trabecular bone loss, cortical porosity and bone marrow adipose tissue (BMAT) accumulation in distal femurs of 2‐ and 4‐month‐old female Tg5516, Tg5519 and wild‐type littermates through microtomographic analysis. (a) Representative 3D reconstructed longitudinal images of trabecular (orange) and cortical bone (grey), at metaphysis (scale bar = 500 μm) and (b) quantification of trabecular bone volume fraction (BV/TV, %) and separation (Tb.S). (c) Representative 3D reconstructed images of BMAT upon osmium staining at metaphyseal region (scale bar = 500 μm) and (d) quantitation of BMAT with adipose volume fraction (Ad.V/Ma.V,%).
Fig. 1. Comparison of staining behaviour of the evaluated CESAs (single and sequential staining). A: Representative CECT images of BMT and MCV, stained with the three CESAs (single staining protocol). Scale bar = 1 mm.
Interplay between bone marrow adiposity and bone resorption in RANKL‐mediated modelled osteoporosis
Journal of Cellular Physiology, Volume 239, Issue 12, pp.1-14(e31434). September 15, 2024.
PMCID: PMC11649960 | PMID: 39279218 | doi: 10.1002/jcp.31434
ABSTRACT
Bone marrow adipose tissue (BMAT) accrues in osteoporosis, whereas its contribution to the progression of bone resorption remains insufficiently understood. To understand the mechanisms that promote BMAT expansion in osteoporosis, in the present study, we performed extensive analysis of the spatiotemporal pattern of BMAT expansion during the progression of bone resorption in TgRANKL transgenic mouse models of osteoporosis expressing human RANKL (receptor activator of nuclear factor‐κB ligand). Our results showed that TgRANKL mice of both sexes developed dramatically increased BMAT expansion compared to wild‐type (WT) littermates, that was analogous to the levels of RANKL expression and the severity of the bone loss phenotype. BMAT was formed at close proximity to areas undergoing active bone remodelling and bone resorption, whereas bone resorption preceded BMAT development. Expression analysis in bone fractions demonstrated that BMAT constitutes a major source for RANKL production. Ex vivo analysis of isolated bone marrow stromal cells from TgRANKL mice showed an increased adipogenic differentiation capacity compared to WT, while osteoclast supernatants further exaggerated adipogenesis, supporting a critical role of the osteoclast‐derived secretome in the differentiation of bone marrow adipocytes. Furthermore, the effectiveness of an antiosteoporosis treatment in BMAT development was investigated upon treatment of TgRANKL models with the bisphosphonate alendronate. Notably, alendronate effectively improved bone mass and attenuated BMAT expansion, indicating a possible involvement of osteoclasts and bone resorption in BMAT development. On the contrary, inhibition of BMAT with PPARγ antagonists (GW9662 or BADGE) effectively ameliorated BMAT expansion but failed to reverse the osteoporotic phenotype of TgRANKL mice. Overall, our data demonstrate that TgRANKL mice constitute unique genetic mouse models for investigating the pathogenic mechanisms that regulate the development and expansion of BMAT in osteolytic diseases.
© 2024 The Author(s). Journal of Cellular Physiology published by Wiley Periodicals LLC.

Progression of trabecular bone loss, cortical porosity and bone marrow adipose tissue (BMAT) accumulation in distal femurs of 2‐ and 4‐month‐old female Tg5516, Tg5519 and wild‐type littermates through microtomographic analysis. (a) Representative 3D reconstructed longitudinal images of trabecular (orange) and cortical bone (grey), at metaphysis (scale bar = 500 μm) and (b) quantification of trabecular bone volume fraction (BV/TV, %) and separation (Tb.S). (c) Representative 3D reconstructed images of BMAT upon osmium staining at metaphyseal region (scale bar = 500 μm) and (d) quantitation of BMAT with adipose volume fraction (Ad.V/Ma.V,%).
Evaluation of romosozumab’s effects on bone marrow adiposity in postmenopausal osteoporotic women: results from the FRAME bone biopsy sub-study
- 1 Univ Lyon, INSERM, UMR 1033, F-69008, Lyon, France.
- 2 UCB Pharma, Brussels, Belgium.
- 3 Amgen Inc, Thousand Oaks, CA, United States.
Journal of Bone and Mineral Research, Volume 39, Issue 9, September 2024, Pages 1278–1283
PMID: 39023227 | https://pubs.acs.org/doi/pdf/10.1021/ | DOI: 10.1093/jbmr/zjae118
ABSTRACT
Romosozumab, a humanized monoclonal antibody that binds and inhibits sclerostin, produces a marked increase in bone formation with a concomitant decreased bone resorption. This transient rise in bone formation in the first 2 months of treatment is mainly due to an increased modeling-based bone formation. This requires the recruitment and differentiation of osteoblasts, one possibility being a preferential switch in commitment of precursors to osteoblasts over adipocytes. The purpose of this study was to analyze the marrow adiposity in transiliac bone biopsies at months 2 or 12 from the FRAME biopsy sub-study in patients receiving romosozumab or placebo. The total adipocyte area, number, and density were measured on the total cancellous bone area. The size and shape at the individual adipocyte level were assessed including the mean adipocyte area, perimeter, min and max diameters, and aspect ratio. No significant difference in total adipocyte area, number, or density between placebo and romosozumab groups was observed at months 2 and 12, and no difference was observed between 2 and 12 months. After 2 or 12 months, romosozumab did not modify the size or shape of the adipocytes. No relationship between the adipocyte parameters and the dynamic parameters of bone formation could be evidenced. In conclusion, based on the analysis of a small number of biopsies, no effect of romosozumab on bone marrow adiposity of iliac crest was identified after 2 and 12 months suggesting that the modeling-based formation observed at month 2 was not due to a preferential commitment of the precursor to osteoblast over adipocyte cell lines but may result from a reactivation of bone lining cells and from a progenitor pool independent of the marrow adipocyte population.
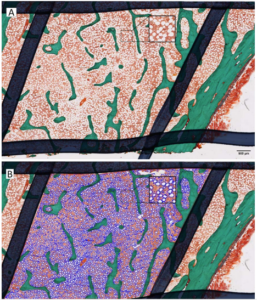
adipocytes. High power image in the square detection and measurement of total adipocyte area by automatic image analyzer (B, blue).
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research. All rights reserved.
Withaferin A Ameliorated the Bone Marrow Fat Content in Obese Male Mice by Favoring Osteogenesis in Bone Marrow Mesenchymal Stem Cells and Preserving the Bone Mineral Density
1 Endocrinology Division, CSIR-Central Drug Research Institute, Lucknow 226031, India; 2 Academy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India; 3Pharmanza Herbal Pvt. Ltd., Anand, Gujarat 388435, India; *Corresponding Author.
PMID: 39296264 | PMCID: PMC11406682 | https://pubs.acs.org/doi/pdf/10.1021/acsptsci.3c00356 | DOI: 10.1021/acsptsci.3c00356
ABSTRACT
Obesity and osteoporosis are two prevalent conditions that are becoming increasingly common worldwide, primarily due to aging populations, imbalanced energy intake, and sedentary lifestyles. Obesity, characterized by excessive fat accumulation, and osteoporosis, marked by reduced bone density and increased fracture risk, are often interconnected. High-fat diets (HFDs) can exacerbate both conditions by promoting bone marrow adiposity and bone loss. The effect of WFA on the osteogenesis and adipogenesis was studied on the C3H10T1/2 cell line and bone marrow mesenchymal stem cells (BM-MSCs) isolated from mice. We used oil red O and alkaline phosphatase (ALP) staining to observe adipogenesis and osteogenesis, respectively, in MSCs. Real-time PCR and Western blot analyses were used to study the molecular effects of WFA on MSCs. We employed micro-CT to analyze the bone microarchitecture, bone mineral density (BMD), and abdominal fat mass in male mice. We have used osmium tetroxide (OsO4) staining to study the bone marrow fat. WFA induced the C3H10T1/2 cell line and BM-MSCs toward osteogenic lineage as evidenced by the higher ALP activity. WFA also downregulated the lipid droplet formation and adipocyte specific genes in MSCs. In the in vivo study, WFA also suppressed the bone catabolic effects of the HFD and maintained the bone microarchitecture and BMD in WFA-treated animals. The bone marrow adipose tissue was reduced in the tibia of WFA-treated groups in comparison with only HFD-fed animals. Withaferin A was able to improve the bone microarchitecture and BMD by committing BM-MSCs toward osteogenic differentiation and reducing marrow adiposity. The findings of this study could provide valuable insights into the therapeutic potential of Withaferin A for combating bone marrow obesity and osteoporosis, particularly in the context of diet-induced metabolic disturbances.


Copyright © 2024 American Chemical Society
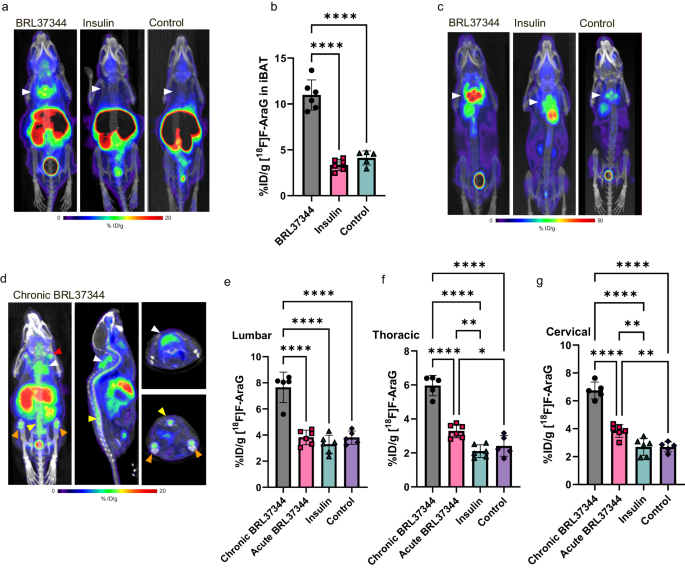
[18F]F-AraG imaging reveals association between neuroinflammation and brown- and bone marrow adipose tissue
1 CellSight Technologies Incorporated, San Francisco, CA, USA. jlevi@cellsighttech.com. 2 Department of Physical Therapy and Rehabilitation Science, University of California San Francisco, San Francisco, CA, USA. 3 Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, USA. 4 Mallinckrodt Institute of Radiology, Washington University in St. Louis, St. Louis, MO, USA. 5 Division of Experimental Medicine, University of California San Francisco, San Francisco, CA, USA. 6 Division of Endocrinology, Department of Internal Medicine, University of California Davis School of Medicine, Davis, CA, USA. 7 Dana-Farber Cancer Institute, Boston, MA, USA. 8 CellSight Technologies Incorporated, San Francisco, CA, USA. 9 Division of HIV, ID and Global Medicine, University of California San Francisco, San Francisco, CA, USA. 10 Molecular Cancer Imaging Facility, Dana-Farber Cancer Institute, Boston, MA, USA. 11 Lurie Family Imaging Center, Dana-Farber Cancer Institute, Boston, MA, USA. 12 Department of Radiology, Stanford University, Palo Alto, CA, USA.
PMID: 38951146 | PMCID: PMC11217368 | https://rdcu.be/dVDAl DOI:10.1038/s42003-024-06494-x
ABSTRACT
Brown and brown-like adipose tissues have attracted significant attention for their role in metabolism and therapeutic potential in diabetes and obesity. Despite compelling evidence of an interplay between adipocytes and lymphocytes, the involvement of these tissues in immune responses remains largely unexplored. This study explicates a newfound connection between neuroinflammation and brown- and bone marrow adipose tissue. Leveraging the use of [18F]F-AraG, a mitochondrial metabolic tracer capable of tracking activated lymphocytes and adipocytes simultaneously, we demonstrate, in models of glioblastoma and multiple sclerosis, the correlation between intracerebral immune infiltration and changes in brown- and bone marrow adipose tissue. Significantly, we show initial evidence that a neuroinflammation-adipose tissue link may also exist in humans. This study proposes the concept of an intricate immuno-neuro-adipose circuit, and highlights brown- and bone marrow adipose tissue as an intermediary in the communication between the immune and nervous systems. Understanding the interconnectedness within this circuitry may lead to advancements in the treatment and management of various conditions, including cancer, neurodegenerative diseases and metabolic disorders.
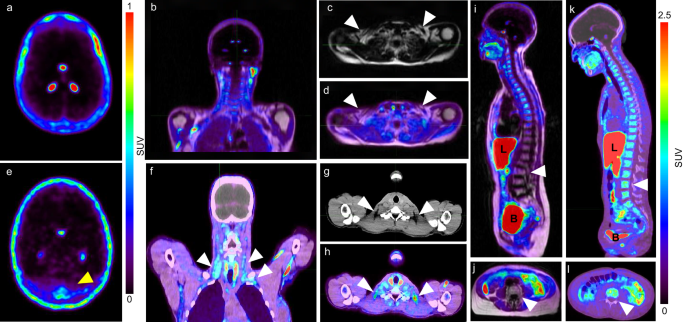
© 2024. The Author(s).
PCLAF induces bone marrow adipocyte senescence and contributes to skeletal aging
Lingqi Xie1, Yalun Cheng1, Biao Hu1, Xin Chen 1 , Yuze An 1 , Zhuying Xia 1 , Guangping Cai1 , Changjun Li 1,2,3 and Hui Peng1
2 – National Clinical Research Centerfor Geriatric Disorders, Xiangya Hospital, Changsha, Hunan 410008, China and
3 – Key Laboratory of Organ Injury, Aging and Regenerative Medicine of Hunan Province, Changsha,Hunan 410008, China
Bone Research, volume 12, Article number: 38 (July 4, 2024)
Bone marrow adipocytes (BMAds) affect bone homeostasis, but the
mechanism remains unclear. Here, we showed that exercise inhibited PCNA
clamp-associated factor (PCLAF) secretion from the bone marrow
macrophages to inhibit BMAds senescence and thus alleviated skeletal
aging. The genetic deletion of PCLAF in macrophages inhibited BMAds
senescence and delayed skeletal aging. In contrast, the transplantation
of PCLAF-mediated senescent BMAds into the bone marrow of healthy mice
suppressed bone turnover. Mechanistically, PCLAF bound to the ADGRL2
receptor to inhibit AKT/mTOR signaling that triggered BMAds senescence
and subsequently spread senescence among osteogenic and osteoclastic
cells. Of note, we developed a PCLAF-neutralizing antibody and showed
its therapeutic effects on skeletal health in old mice. Together, these
findings identify PCLAF as an inducer of BMAds senescence and provide a
promising way to treat age-related osteoporosis.
https://doi.org/10.1038/s41413-024-00337-5 | https://rdcu.be/dTdea

Targeting adipocyte ESRRA promotes osteogenesis and vascular formation in adipocyte-rich bone marrow
Tongling Huang1, Zhaocheng Lu1, Zihui Wang1,2, Lixin Cheng3, Lu Gao1,2, Jun Gao1, Ning Zhang4, Chang-An Geng5, Xiaoli Zhao1, Huaiyu Wang1, Chi-Wai Wong6, Kelvin W K Yeung7, Haobo Pan1, William Weijia Lu8, Min Guan9,10
1 Research Center for Human Tissues and Organs Degeneration, Institute of Biomedicine and Biotechnology, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China. 2 University of Chinese Academy of Sciences, Beijing, China. 3 Guangdong Provincial Clinical Research Center for Geriatrics, Shenzhen Clinical Research Center for Geriatrics, Shenzhen People’s Hospital, Shenzhen, China. 4 Neuroscience Center, Shantou University Medical College, Shantou, China. 5 State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China. 6 Guangzhou Huazhen Biosciences, Guangzhou, China. 7 Department of Orthopaedics and Traumatology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China. 8 Faculty of Pharmaceutical Sciences, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China. 9 Research Center for Human Tissues and Organs Degeneration, Institute of Biomedicine and Biotechnology, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China. 10 University of Chinese Academy of Sciences, Beijing, China. min.guan@siat.ac.cn.
PMID: 38704393 | PMCID: PMC11069533 | DOI: 10.1038/s41467-024-48255-8 | https://rdcu.be/dVDVp
Abstract
Excessive bone marrow adipocytes (BMAds) accumulation often occurs under diverse pathophysiological conditions associated with bone deterioration. Estrogen-related receptor α (ESRRA) is a key regulator responding to metabolic stress. Here, we show that adipocyte-specific ESRRA deficiency preserves osteogenesis and vascular formation in adipocyte-rich bone marrow upon estrogen deficiency or obesity. Mechanistically, adipocyte ESRRA interferes with E2/ESR1 signaling resulting in transcriptional repression of secreted phosphoprotein 1 (Spp1); yet positively modulates leptin expression by binding to its promoter. ESRRA abrogation results in enhanced SPP1 and decreased leptin secretion from both visceral adipocytes and BMAds, concertedly dictating bone marrow stromal stem cell fate commitment and restoring type H vessel formation, constituting a feed-forward loop for bone formation. Pharmacological inhibition of ESRRA protects obese mice against bone loss and high marrow adiposity. Thus, our findings highlight a therapeutic approach via targeting adipocyte ESRRA to preserve bone formation especially in detrimental adipocyte-rich bone milieu.
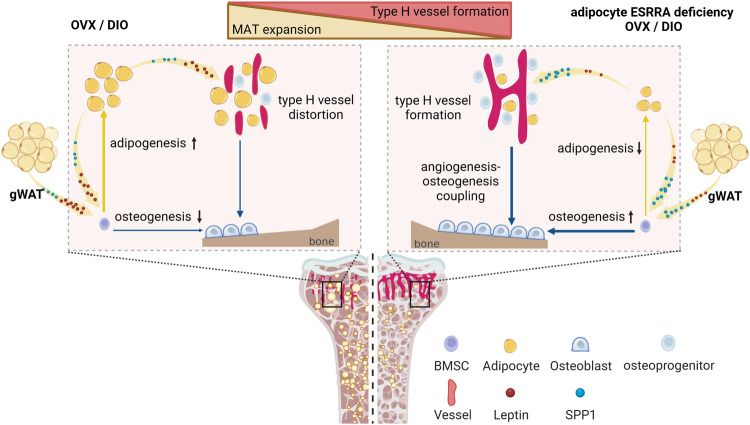
Fig. 9. Schematic diagram showing adipocyte ESRRA deficiency preserves osteogenesis and vascular formation in adipocyte-rich bone marrow via oppositely modulating lepin and SPP1. - © 2024. The Author(s).
Mapping the cellular biogeography of human bone marrow niches using single-cell transcriptomics and proteomic imaging
Shovik Bandyopadhyay, Michael P. Duffy, Kyung Jin Ahn, Jonathan H. Sussman, Minxing Pang, David Smith, Gwendolyn Duncan, Iris Zhang, Jeffrey Huang, Yulieh Lin, Barbara Xiong, Tamjid Imtiaz, Chia-Hui Chen, Anusha Thadi, Changya Chen, Jason Xu, Melissa Reichart, Zachary Martinez, Caroline Diorio, Chider Chen, Vinodh Pillai, Oraine Snaith, Derek Oldridge, Siddharth Bhattacharyya, Ivan Maillard, Martin Carroll, Charles Nelson, Ling Qin, Kai Tan
Non-hematopoietic cells are essential contributors to hematopoiesis. However, heterogeneity and spatial organization of these cells in human bone marrow remain largely uncharacterized. We used single-cell RNA sequencing (scRNA-seq) to profile 29,325 non-hematopoietic cells and discovered nine transcriptionally distinct subtypes. We simultaneously profiled 53,417 hematopoietic cells and predicted their interactions with non-hematopoietic subsets. We employed co-detection by indexing (CODEX) to spatially profile over 1.2 million cells. We integrated scRNA-seq and CODEX data to link predicted cellular signaling with spatial proximity. Our analysis revealed a hyperoxygenated arterio-endosteal neighborhood for early myelopoiesis, and an adipocytic localization for early hematopoietic stem and progenitor cells (HSPCs). We used our CODEX atlas to annotate new images and uncovered mesenchymal stromal cell (MSC) expansion and spatial neighborhoods co-enriched for leukemic blasts and MSCs in acute myeloid leukemia (AML) patient samples. This spatially resolved, multiomic atlas of human bone marrow provides a reference for investigation of cellular interactions that drive hematopoiesis.
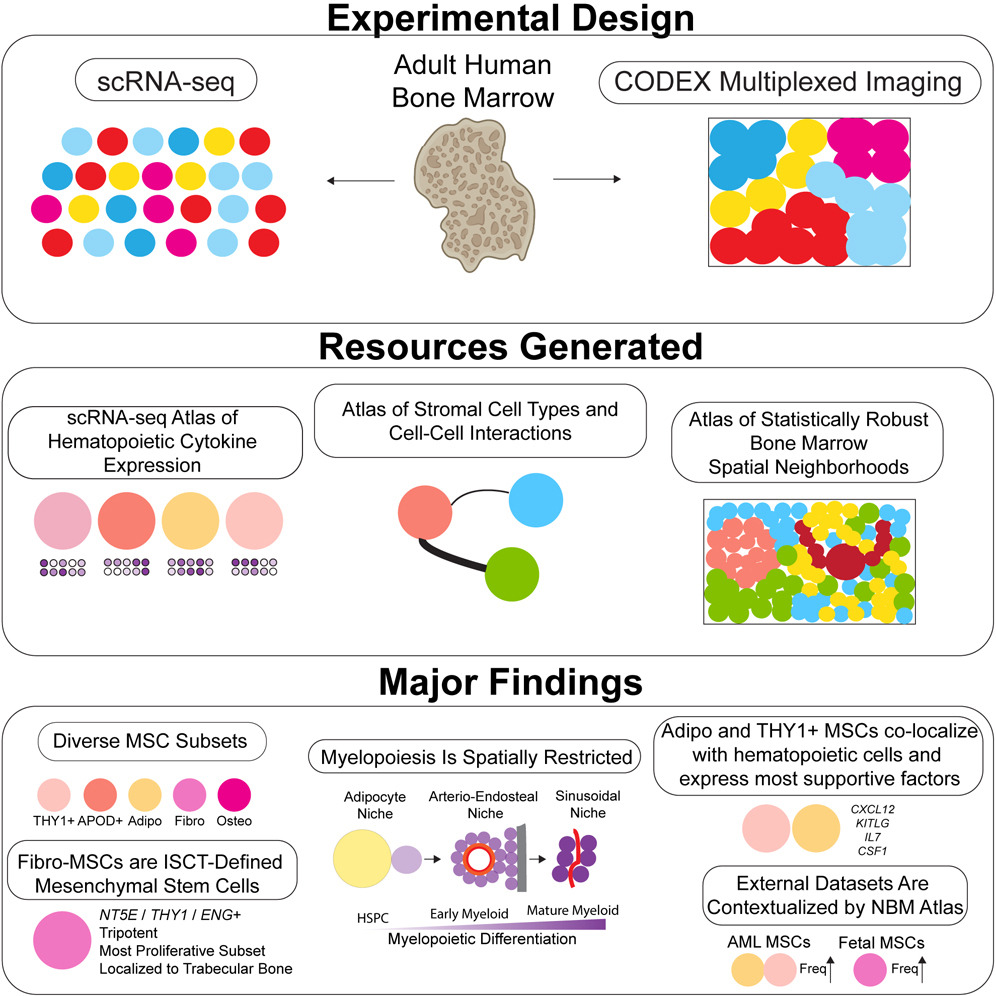
A novel deep learning method for large-scale analysis of bone marrow adiposity using UK Biobank Dixon MRI data
David M. Morris, Chengjia Wang, Giorgos Papanastasiou, Calum D. Gray, Wei Xu, Samuel Sjöström, Sammy Badr, Julien Paccou, Scott IK Semple, Tom MacGillivray, William P. Cawthorn
Background: Bone marrow adipose tissue (BMAT) represents > 10% fat mass in healthy humans and can be measured by magnetic resonance imaging (MRI) as the bone marrow fat fraction (BMFF). Human MRI studies have identified several diseases associated with BMFF but have been relatively small scale. Population-scale studies therefore have huge potential to reveal BMAT’s true clinical relevance. The UK Biobank (UKBB) is undertaking MRI of 100,000 participants, providing the ideal opportunity for such advances.
Objective: To establish deep learning for high-throughput multi-site BMFF analysis from UKBB MRI data.
Materials and methods: We studied males and females aged 60–69. Bone marrow (BM) segmentation was automated using a new lightweight attention-based 3D U-Net convolutional neural network that improved segmentation of small structures from large volumetric data. Using manual segmentations from 61–64 subjects, the models were trained to segment four BM regions of interest: the spine (thoracic and lumbar vertebrae), femoral head, total hip and femoral diaphysis. Models were tested using a further 10–12 datasets per region and validated using datasets from 729 UKBB participants. BMFF was then quantified and pathophysiological characteristics assessed, including site- and sex-dependent differences and the relationships with age, BMI, bone mineral density, peripheral adiposity, and osteoporosis.
Results: Model accuracy matched or exceeded that for conventional U-Nets, yielding Dice scores of 91.2% (spine), 94.5% (femoral head), 91.2% (total hip) and 86.6% (femoral diaphysis). One case of severe scoliosis prevented segmentation of the spine, while one case of Non-Hodgkin Lymphoma prevented segmentation of the spine, femoral head and total hip because of T2 signal depletion; however, successful segmentation was not disrupted by any other pathophysiological variables. The resulting BMFF measurements confirmed expected relationships between BMFF and age, sex and bone density, and identified new site- and sex-specific characteristics.
Conclusions: We have established a new deep learning method for accurate segmentation of small structures from large volumetric data, allowing high-throughput multi-site BMFF measurement in the UKBB. Our findings reveal new pathophysiological insights, highlighting the potential of BMFF as a novel clinical biomarker. Applying our method across the full UKBB cohort will help to reveal the impact of BMAT on human health and disease.
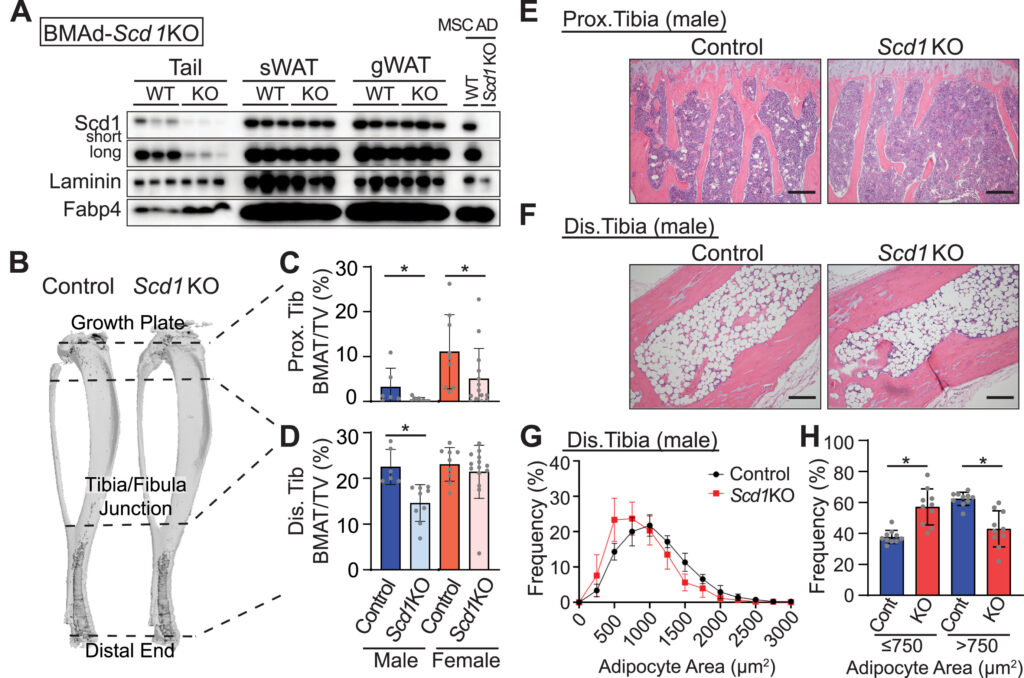
Scd1 and monounsaturated lipids are required
for autophagy and survival of adipocytes
Hiroyuki Mori, Sydney K. Peterson, Rachel C. Simmermon, Katherine A. Overmyer, Akira Nishii, Emma Paulsson, Ziru Li, Annie Jen, Romina M. Uranga, Jessica N. Maung, Warren T. Yacawych, Kenneth T. Lewis, Rebecca L. Schill, Taryn Hetrick, Ryo Seino, Ken Inoki, Joshua J. Coon, Ormond A. MacDougald
Objective: Exposure of adipocytes to ‘cool’ temperatures often found in the periphery of the body induces expression of Stearoyl-CoA Desaturase-1 (Scd1), an enzyme that converts saturated fatty acids to monounsaturated fatty acids. The goal of this study is to further investigate the roles of Scd in adipocytes.
Method: In this study, we employed Scd1 knockout cells and mouse models, along with pharmacological Scd1 inhibition to dissect the enzyme’s function in adipocyte physiology.
Results: Our study reveals that production of monounsaturated lipids by Scd1 is necessary for fusion of autophagosomes to lysosomes and that with a Scd1-deficiency, autophagosomes accumulate. In addition, Scd1-deficiency impairs lysosomal and autolysosomal acidification resulting in vacuole accumulation and eventual cell death. Blocking autophagosome formation or supplementation with monounsaturated fatty acids maintains vitality of Scd1-deficient adipocytes.
Conclusion: This study demonstrates the indispensable role of Scd1 in adipocyte survival, with its inhibition in vivo triggering autophagy-dependent cell death and its depletion in vivo leading to the loss of bone marrow adipocytes.
Bone marrow adipocytes fuel emergency hematopoiesis after myocardial infarction
Shuang Zhang, Alexandre Paccalet, David Rohde, Sebastian Cremer, Maarten Hulsmans, I-Hsiu Lee, Kyle Mentkowski, Jana Grune, Maximilian J. Schloss, Lisa Honold, Yoshiko Iwamoto, Yi Zheng, Miriam A. Bredella, Colleen Buckless, Brian Ghoshhajra, Vikas Thondapu, Anja M. van der Laan, Jan J. Piek, Hans W. M. Niessen, Fabio Pallante, Raimondo Carnevale, Sara Perrotta, Daniela Carnevale, Oriol Iborra-Egea, Christian Muñoz-Guijosa, Carolina Galvez-Monton, Antoni Bayes-Genis, Charles Vidoudez, Sunia A. Trauger, David T. Scadden, Filip K. Swirski, Michael A. Moskowitz, Kamila Naxerova & Matthias Nahrendorf
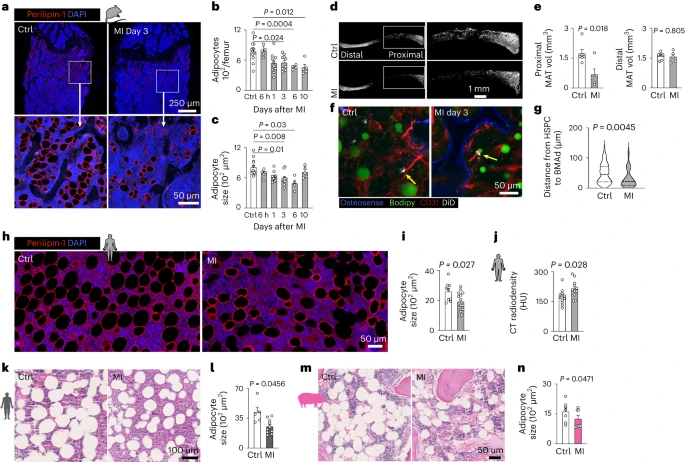
After myocardial infarction (MI), emergency hematopoiesis produces inflammatory myeloid cells that accelerate atherosclerosis and promote heart failure. Because the balance between glycolysis and mitochondrial metabolism regulates hematopoietic stem cell homeostasis, metabolic cues may influence emergency myelopoiesis. Here we show, in humans and female mice, that hematopoietic progenitor cells increase fatty acid metabolism after MI. Blockade of fatty acid oxidation by deleting carnitine palmitoyltransferase (Cpt1a) in hematopoietic cells of Vav1Cre/+Cpt1afl/fl mice limited hematopoietic progenitor proliferation and myeloid cell expansion after MI. We also observed reduced bone marrow adiposity in humans, pigs and mice after MI. Inhibiting lipolysis in adipocytes using AdipoqCreERT2Atglfl/fl mice or local depletion of bone marrow adipocytes in AdipoqCreERT2iDTR mice also curbed emergency hematopoiesis. Furthermore, systemic and regional sympathectomy prevented bone marrow adipocyte shrinkage after MI. These data establish a critical role for fatty acid metabolism in post-MI emergency hematopoiesis.
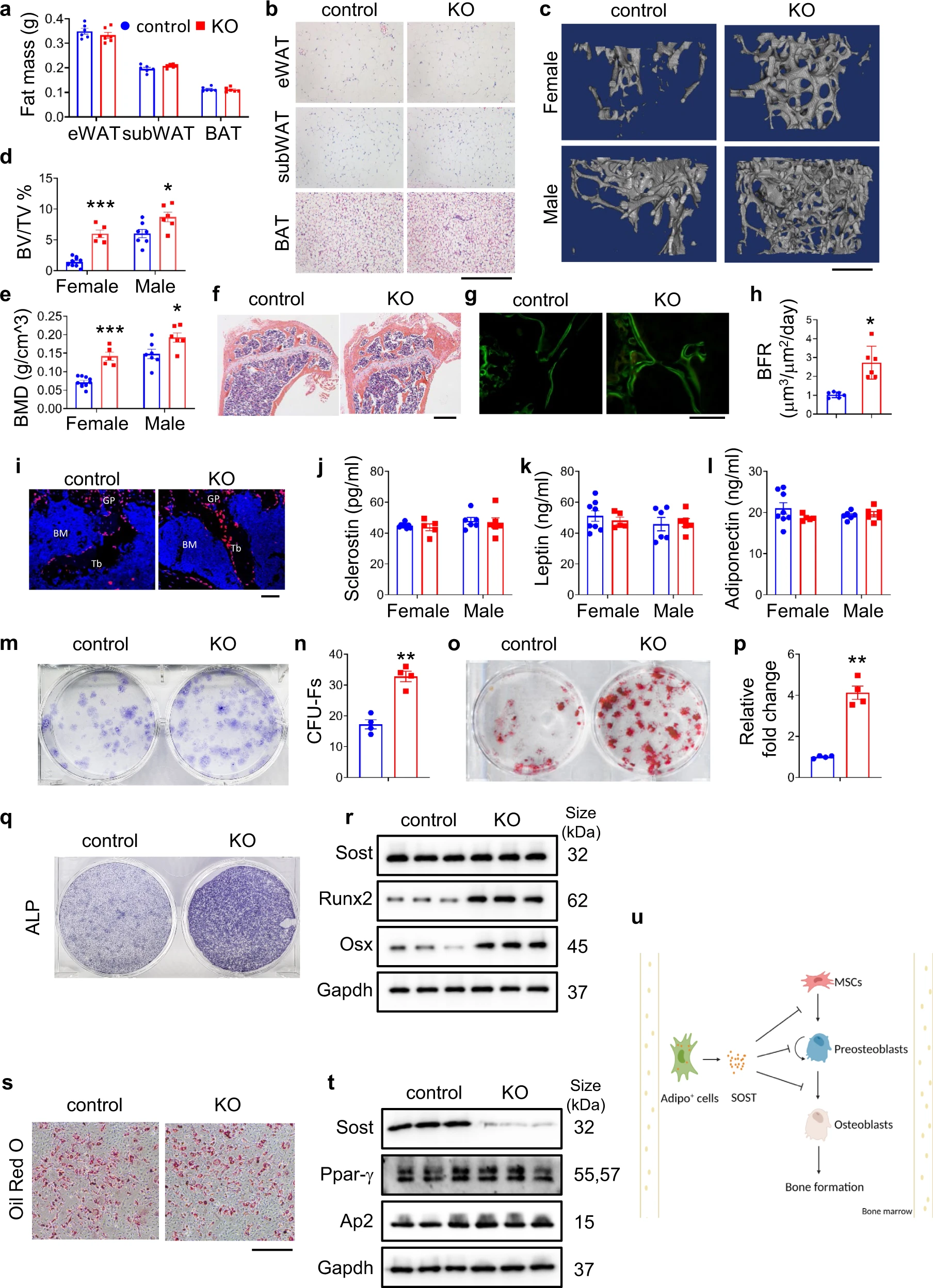
Bone marrow adipoq+ cell population controls bone mass via sclerostin in mice
Huanqing Gao, Yiming Zhong, Sixiong Lin, Qinnan Yan, Xuenong Zou, Guozhi Xiao
The comorbidity of obesity and osteoporosis illustrates the communication and coordination of adipose and bone tissues. Leptin and adiponectin derived from adipocytes regulate osteoblast formation and function to impact bone mass through direct and indirect mechanisms. It is known that bone marrow adipocytes (BMA) can control bone mass by modulating the bone morphogenetic protein (BMP) and other signaling pathways. BMAs can secret soluble factors, which impact osteoblasts, osteoclasts, and osteocytes. Sclerostin is a potent inhibitor of bone acquisition that antagonizes Wnt/β-catenin signaling. Deleting sclerostin was recently reported to protect against cardiovascular disease. Furthermore, neutralizing monoclonal antibodies against sclerostin increase bone mass and are utilized to treat osteoporosis. Previous studies revealed that global ablation of sclerostin increased both trabecular and cortical bone mass and that sclerostin produced by the osteocytes located in the bone matrix negatively regulated bone mass in mice. However, it is not known whether sclerostin derived from other cell types also contributes to bone formation. Hence, we have explored the contribution of adiponectin-expressing cells-derived sclerostin in control of bone mass by ablating of Sost gene, which encodes sclerostin, using the Adipoq-Cre that mainly targets adipose lineage cells.
Omega-3 PUFAs prevent bone impairment and bone marrow adiposity in mouse model of obesity
Andrea Benova, Michaela Ferencakova, Kristina Bardova, Jiri Funda, Jan Prochazka, Frantisek Spoutil, Tomas Cajka, Martina Dzubanova, Tim Balcaen, Greet Kerckhofs, Wouter Willekens, G. Harry van Lenthe, Arzuv Charyyeva, Glenda Alquicer, Alena Pecinova, Tomas Mracek, Olga Horakova, Roman Coupeau, Morten Svarer Hansen, Martin Rossmeisl, Jan Kopecky, Michaela Tencerova
Obesity adversely affects bone and fat metabolism in mice and humans. Omega-3 polyunsaturated fatty acids (omega-3 PUFAs) have been shown to improve glucose metabolism and bone homeostasis in obesity. However, the impact of omega-3 PUFAs on bone marrow adipose tissue (BMAT) and bone marrow stromal cell (BMSC) metabolism has not been intensively studied yet. In the present study we demonstrated that omega-3 PUFA supplementation in high fat diet (HFD + F) improved bone parameters, mechanical properties along with decreased BMAT in obese mice when compared to the HFD group. Primary BMSCs isolated from HFD + F mice showed decreased adipocyte and higher osteoblast differentiation with lower senescent phenotype along with decreased osteoclast formation suggesting improved bone marrow microenvironment promoting bone formation in mice. Thus, our study highlights the beneficial effects of omega-3 PUFA-enriched diet on bone and cellular metabolism and its potential use in the treatment of metabolic bone diseases.
Cellular plasticity of the bone marrow niche promotes hematopoietic stem cell regeneration
Hiroyuki Hirakawa, Longfei Gao, Daniel Naveed Tavakol, Gordana Vunjak-Novakovic, Lei Ding
Csf1 from marrow adipogenic precursors is required for osteoclast formation and hematopoiesis in bone
Leilei Zhong Jiawei Lu, Jiankang Fang, Lutian Yao,Wei Yu, Tao Gui, Michael Duffy, Nicholas Holdreith, Catherine A Bautista, Xiaobin Huang, Shovik Bandyopadhyay, Kai Tan, Chider Chen, Yongwon Choi, Jean X Jiang, Shuying Yang, Wei Tong, Nathanial Dyment, Ling Qin
Bone marrow Adipoq-lineage progenitors are a major cellular source of M-CSF that dominates bone marrow macrophage development, osteoclastogenesis, and bone mass
Kazuki Inoue, Yongli Qin, Yuhan Xia, Jie Han, Ruoxi Yuan, Jun Sun, Ren Xu, Jean X Jiang, Matthew B Greenblatt, Baohong Zhao
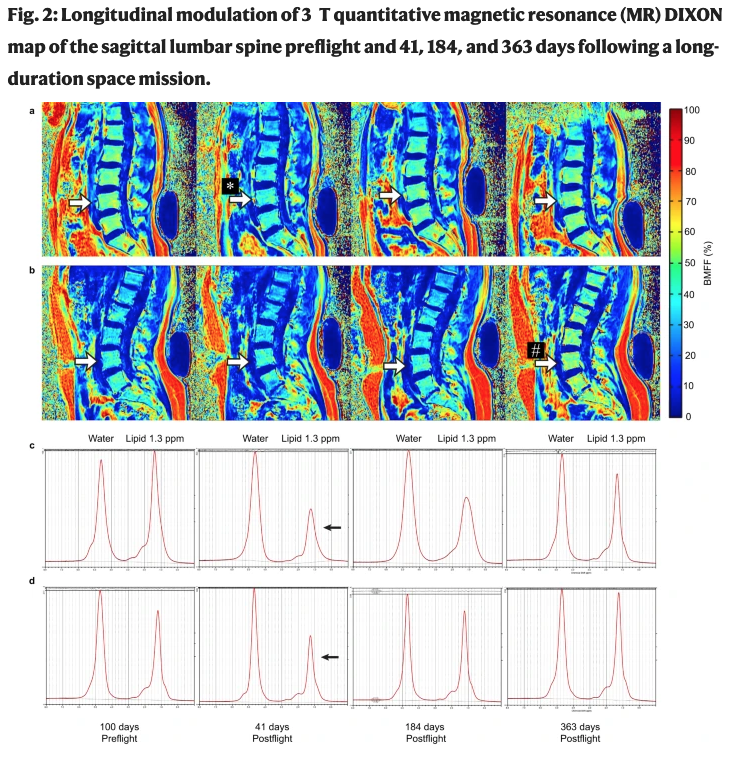
Bone marrow adiposity modulation after long duration spaceflight in astronauts
Tammy Liu, Gerd Melkus, Tim Ramsay, Adnan Sheikh, Odette Laneuville, Guy Trudel
Bone marrow adipocytes drive the development of tissue invasive Ly6Chigh monocytes during obesity
Parastoo Boroumand, David C Prescott, Tapas Mukherjee, Philip J Bilan, Michael Wong, Jeff Shen, Ivan Tattoli, Yuhuan Zhou, Angela Li, Tharini Sivasubramaniyam, Nancy Shi, Lucie Y Zhu, Zhi Liu, Clinton Robbins, Dana J Philpott, Stephen E Girardin, Amira Klip
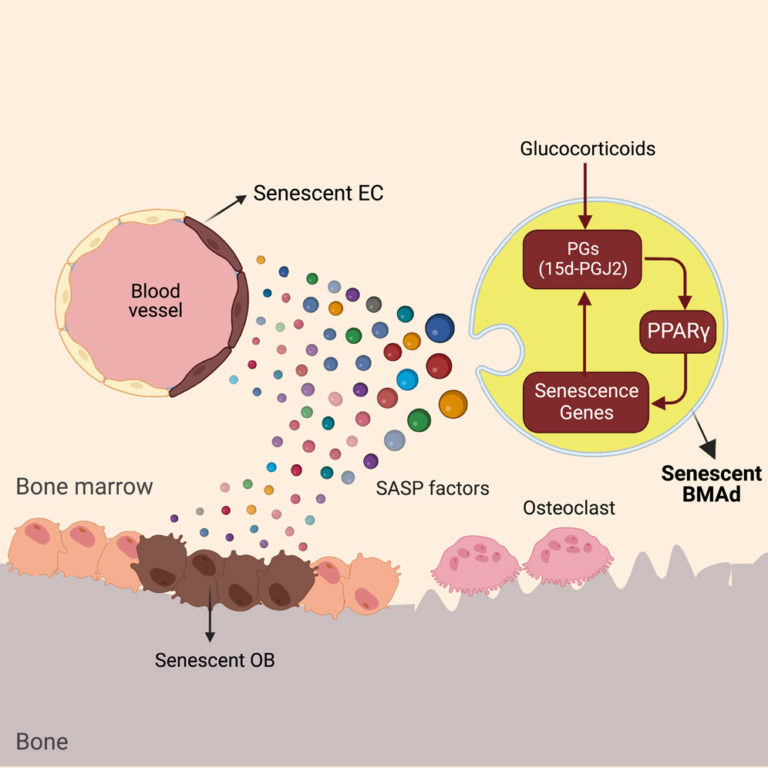
Oxylipin-PPARγ-initiated adipocyte senescence propagates secondary senescence in the bone marrow
Accordion Content
Constitutive bone marrow adipocytes suppress local bone formation
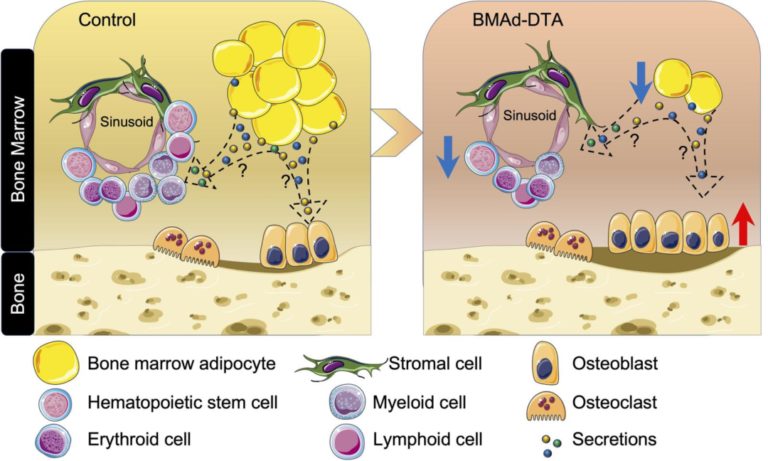
GSαR201C and estrogen reveal different subsets of bone marrow adiponectin expressing osteogenic cells
A mechanosensitive lipolytic factor in the bone marrow promotes osteogenesis and lymphopoiesis
Single-cell transcriptomics of LepR-positive skeletal cells reveals heterogeneous stress-dependent stem and progenitor pools
Chunyang Mo, Jingxin Guo, Jiachen Qin, Xiaoying Zhang, Yuxi Sun, Hanjing Wei, Dandan Cao, Yiying Zhang, Chengchen Zhao, Yanhong Xiong, Yong Zhang, Yao Sun, Li Shen, Rui Yue
Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits
Ziru Li, Emily Bowers, Junxiong Zhu, View ORCID ProfileHui Yu, Julie Hardij, Devika P. Bagchi, Hiroyuki Mori, Kenneth T. Lewis, Katrina Granger, Rebecca L. Schill, Steven M. Romanelli, Simin Abrishami, Kurt D. Hankenson, View ORCID ProfileKanakadurga Singer, View ORCID ProfileClifford J. Rosen, View ORCID ProfileOrmond A. MacDougald
To investigate roles for bone marrow adipocyte (BMAd) lipolysis in bone homeostasis, we created a BMAd-specific Cre mouse model in which we knocked out adipose triglyceride lipase (ATGL, Pnpla2). BMAd-Pnpla2-/- mice have impaired BMAd lipolysis, and increased size and number of BMAds at baseline. Although energy from BMAd lipid stores is largely dispensable when mice are fed ad libitum, BMAd lipolysis is necessary to maintain myelopoiesis and bone mass under caloric restriction. BMAd-specific Pnpla2 deficiency compounds the effects of caloric restriction on loss of trabecular bone, likely due to impaired osteoblast expression of collagen genes and reduced osteoid synthesis. RNA sequencing analysis of bone marrow adipose tissue reveals that caloric restriction induces dramatic elevations in extracellular matrix organization and skeletal development genes, and energy from BMAd is required for these adaptations. BMAd-derived energy supply is also required for bone regeneration upon injury, and maintenance of bone mass with cold exposure.
Exercise Increases Bone in SEIPIN Deficient Lipodystrophy, Despite Low Marrow Adiposity
Cody McGrath, Sarah E. Little-Letsinger, Jeyantt Srinivas Sankaran, Buer Sen, Zhihui Xie, Martin A. Styner, Xiaopeng Zong, Weiqin Chen, Janet Rubin, Eric L. Klett, Rosalind A. Coleman and Maya Styner
Exercise, typically beneficial for skeletal health, has not yet been studied in lipodystrophy, a condition characterized by paucity of white adipose tissue, with eventual diabetes, and steatosis. We applied a mouse model of global deficiency of Bscl2 (SEIPIN), required for lipid droplet formation. Male twelve-week-old B6 knockouts (KO) and wild type (WT) littermates were assigned six-weeks of voluntary, running exercise (E) versus non-exercise (N=5-8). KO weighed 14% less than WT (p=0.01) and exhibited an absence of epididymal adipose tissue; KO liver Plin1 via qPCR was 9-fold that of WT (p=0.04), consistent with steatosis. Bone marrow adipose tissue (BMAT), unlike white adipose, was measurable, although 40.5% lower in KO vs WT (p=0.0003) via 9.4T MRI/advanced image analysis. SEIPIN ablation’s most notable effect marrow adiposity was in the proximal femoral diaphysis (-56% KO vs WT, p=0.005), with relative preservation in KO-distal-femur. Bone via μCT was preserved in SEIPIN KO, though some quality parameters were attenuated. Running distance, speed, and time were comparable in KO and WT. Exercise reduced weight (-24% WT-E vs WT p<0.001) but not in KO. Notably, exercise increased trabecular BV/TV in both (+31%, KO-E vs KO, p=0.004; +14%, WT-E vs WT, p=0.006). The presence and distribution of BMAT in SEIPIN KO, though lower than WT, is unexpected and points to a uniqueness of this depot. That trabecular bone increases were achievable in both KO and WT, despite a difference in BMAT quantity/distribution, points to potential metabolic flexibility during exercise-induced skeletal anabolism.
Saturated and Unsaturated Bone Marrow Lipids Have Distinct Effects on Bone Density and Fracture Risk in Older Adults
Gina N. Woods,Susan K. Ewing,Anne L. Schafer,Vilmundur Gudnason,Sigurdur Sigurdsson,Thomas Lang,Trisha F. Hue,Deborah M. Kado,Eric Vittinghoff,Clifford Rosen,Xiaojuan Li,Ann V. Schwartz
Gina N. Woods,Susan K. Ewing,Anne L. Schafer,Vilmundur Gudnason,Sigurdur Sigurdsson,Thomas Lang,Trisha F. Hue,Deborah M. Kado,Eric Vittinghoff,Clifford Rosen,Xiaojuan Li,Ann V. Schwartz
Neural regulation of bone marrow adipose tissue
XiaoZhang, Mohamed G.Hassan, Erica L.Scheller
Best Practice & Research ClinicalEndocrinology & Metabolism. DOI: https://doi.org/10.1182/blood.2020005865
Bone marrow adipose tissue (BMAT) is an important cellular component of the skeleton. Understanding how it is regulated by the nervous system is crucial to the study of bone and bone marrow related diseases. BMAT is innervated by sympathetic and sensory axons in bone and fluctuations in local nerve density and function may contribute to its distinct physiologic adaptations at various skeletal sites. BMAT is directly responsive to adrenergic signals. In addition, neural regulation of surrounding cells may modify BMAT-specific responses, providing many potential avenues for both direct and indirect neural regulation of BMAT metabolism. Lastly, BMAT and peripheral adipose tissues share the same autonomic pathways across the central neuraxis and regulation of BMAT may occur in diverse clinical settings of neurologic and metabolic disease. This review will highlight what is known and unknown about the neural regulation of BMAT and discuss opportunities for future research in the field.
Paper proposed by Alessandro Corsi
Adipsin promotes bone marrow adiposity by priming mesenchymal stem cells
Nicole Aaron, Michael J Kraakman, Qiuzhong Zhou , Qiongming Liu , Samantha Costa, Jing Yang, Longhua Liu, Lexiang Yu, Liheng Wang, Ying He, Lihong Fan, Hiroyuki Hirakawa, Lei Ding, James Lo , Weidong Wang, Baohong Zhao, Edward Guo, Lei Sun , Cliff J Rosen , Li Qiang
Elife, June 2021, doi: 10.7554/eLife.69209
Background:
Marrow adipose tissue (MAT) has been shown to be vital for regulating metabolism and maintaining skeletal homeostasis in the bone marrow (BM) niche. As a reflection of BM remodeling, MAT is highly responsive to nutrient fluctuations, hormonal changes, and metabolic disturbances such as obesity and diabetes mellitus. Expansion of MAT has also been strongly associated with bone loss in mice and humans. However, the regulation of BM plasticity remains poorly understood, as does the mechanism that links changes in marrow adiposity with bone remodeling.
Methods:
We studied deletion of Adipsin, and its downstream effector, C3, in C57BL/6 mice as well as the bone-protected PPARγ constitutive deacetylation 2KR mice to assess BM plasticity. The mice were challenged with thiazolidinedione treatment, calorie restriction, or aging to induce bone loss and MAT expansion. Analysis of bone mineral density and marrow adiposity was performed using a μCT scanner and by RNA analysis to assess adipocyte and osteoblast markers. For in vitro studies, primary bone marrow stromal cells were isolated and subjected to osteoblastogenic or adipogenic differentiation or chemical treatment followed by morphological and molecular analyses. Clinical data was obtained from samples of a previous clinical trial of fasting and high-calorie diet in healthy human volunteers.
Results:
We show that Adipsin is the most upregulated adipokine during MAT expansion in mice and humans in a PPARγ acetylation-dependent manner. Genetic ablation of Adipsin in mice specifically inhibited MAT expansion but not peripheral adipose depots, and improved bone mass during calorie restriction, thiazolidinedione treatment, and aging. These effects were mediated through its downstream effector, complement component C3, to prime common progenitor cells toward adipogenesis rather than osteoblastogenesis through inhibiting Wnt/β-catenin signaling.
Conclusions:
Adipsin promotes new adipocyte formation and affects skeletal remodeling in the BM niche. Our study reveals a novel mechanism whereby the BM sustains its own plasticity through paracrine and endocrine actions of a unique adipokine.
Proposed by Stephanie Lucas
The characterization of distinct populations of murine skeletal cells that have different roles in B lymphopoiesis
Alanna Claire Green , Gavin Tjin , Samuel C Lee , Alistair M Chalk , Lenny Straszkowski , Diannita Kwang , Emma K Baker , Julie M Quach , Takaharu Kimura , Joy Wu , Louise E. Purton
Blood, 2021. DOI: https://doi.org/10.1182/blood.2020005865
Hematopoiesis is extrinsically controlled by cells of the bone marrow microenvironment, including skeletal lineage cells. The identification and subsequent studies of distinct subpopulations of maturing skeletal cells is currently limited due to a lack of methods to isolate these cells. We found that murine Lineage–CD31–Sca-1–CD51+ cells can be divided into four subpopulations using flow cytometry, based on their expression of the platelet derived growth factor receptors ⍺ and β (PDGFR⍺ and PDGFRβ). The use of different skeletal lineage reporters confirmed the skeletal origin of the four populations. Multiplex immunohistochemistry studies revealed that all four populations were localized near the growth plate and trabecular bone and were rarely found near cortical bone regions or in central bone marrow. Functional studies revealed differences in their abundance, colony-forming unit-fibroblast capacity and potential to differentiate into mineralized osteoblasts or adipocytes in vitro. Furthermore, the four populations had distinct gene expression profiles and differential cell surface expression of leptin receptor (LEPR) and vascular cell adhesion molecule 1 (VCAM-1). Interestingly, we discovered that one of these four different skeletal populations showed the highest expression of genes involved in the extrinsic regulation of B lymphopoiesis. This cell population varied in abundance between distinct hematopoietically active skeletal sites, and significant differences in the proportions of B lymphocyte precursors were also observed in these distinct skeletal sites. It also supported pre-B lymphopoiesis in culture. Our method to isolate four distinct maturing skeletal populations will assist in elucidating the roles of distinct skeletal niche cells in regulating hematopoiesis and bone.
Paper proposed by Michaela Reagan
MyelomaModified Adipocytes Exhibit Metabolic Dysfunction and a Senescence-Associated Secretory Phenotype
Heather Fairfield, Amel Dudakovic , Casper M Khatib, Mariah Farrell , Samantha Costa , Carolyne Falank , Maja Hinge , Connor S Murphy , Victoria DeMambro , Jessica A Pettitt , Christine W Lary , Heather E Driscoll Michelle M McDonald , Moustapha Kassem , Clifford Rosen , Thomas L Andersen, Andre J van Wijnen, Abbas Jafari, Michaela R Reagan
Cancer Research, 2021. DOI: 10.1158/0008-5472.CAN-20-1088
Bone marrow adipocytes (BMAd) have recently been implicated in accelerating bone metastatic cancers, such as acute myelogenous leukemia and breast cancer. Importantly, bone marrow adipose tissue (BMAT) expands with aging and obesity, two key risk factors in multiple myeloma disease prevalence, suggesting that BMAds may influence and be influenced by myeloma cells in the marrow. Here, we provide evidence that reciprocal interactions and cross-regulation of myeloma cells and BMAds play a role in multiple myeloma pathogenesis and treatment response. Bone marrow biopsies from patients with multiple myeloma revealed significant loss of BMAT with myeloma cell infiltration of the marrow, whereas BMAT was restored after treatment for multiple myeloma. Myeloma cells reduced BMAT in different preclinical murine models of multiple myeloma and in vitro using myeloma cell-adipocyte cocultures. In addition, multiple myeloma cells altered adipocyte gene expression and cytokine secretory profiles, which were also associated with bioenergetic changes and induction of a senescent-like phenotype. In vivo, senescence markers were also increased in the bone marrow of tumor-burdened mice. BMAds, in turn, provided resistance to dexamethasone-induced cell-cycle arrest and apoptosis, illuminating a new possible driver of myeloma cell evolution in a drug-resistant clone. Our findings reveal that bidirectional interactions between BMAds and myeloma cells have significant implications for the pathogenesis and treatment of multiple myeloma. Targeting senescence in the BMAd or other bone marrow cells may represent a novel therapeutic approach for treatment of multiple myeloma. SIGNIFICANCE: This study changes the foundational understanding of how cancer cells hijack the bone marrow microenvironment and demonstrates that tumor cells induce senescence and metabolic changes in adipocytes, potentially driving new therapeutic directions…
Paper proposed by Erica Scheller
Increased marrow adipogenesis does not contribute to age-dependent appendicular bone loss in female mice
Aging Cell, 2020. doi: 10.1111/acel.13247
Marrow adipocytes and osteoblasts differentiate from common mesenchymal progenitors in a mutually exclusive manner, and diversion of these progenitors toward adipocytes in old age has been proposed to account for the decline in osteoblasts and the development of involutional osteoporosis. This idea has been supported by evidence that thiazolidinedione (TZD)-induced activation of PPARγ, the transcription factor required for adipocyte differentiation, increases marrow fat and causes bone loss. We functionally tested this hypothesis using C57BL/6J mice with conditional deletion of PPARγ from early mesenchymal progenitors targeted by the Prx1-Cre transgene. Using a longitudinal littermate-controlled study design, we observed that PPARγ is indispensable for TZD-induced increase in marrow adipocytes in 6-month-old male mice, and age-associated increase in marrow adipocytes in 22-month-old female mice. In contrast, PPARγ is dispensable for the loss of cortical and trabecular bone caused by TZD or old age. Instead, PPARγ restrains age-dependent development of cortical porosity. These findings do not support the long-standing hypothesis that increased marrow adipocyte differentiation contributes to bone loss in old age but reveal a novel role of mesenchymal cell PPARγ in the maintenance of cortical integrity.
Paper proposed by Eleni Douni
The Journal of Clinical Investigation, 2020. doi: https://doi.org/10.1172/JCI140214.
Bone is maintained by coupled activities of bone-forming osteoblasts/osteocytes and bone-resorbing osteoclasts. Alterations in this relationship can lead to pathologic bone loss, such as osteoporosis. It is well known that osteogenic cells support osteoclastogenesis via production of RANKL. Interestingly, our recently identified bone marrow mesenchymal cell population—marrow adipogenic lineage precursors (MALPs) that form a multi-dimensional cell network in bone—was computationally demonstrated to be the most interactive with monocyte-macrophage lineage cells through high and specific expression of several osteoclast regulatory factors, including RANKL. Using an adipocyte-specific Adipoq-Cre to label MALPs, we demonstrated that mice with RANKL deficiency in MALPs have a drastic increase in trabecular bone mass in long bones and vertebrae starting from 1 month of age, while their cortical bone appears normal. This phenotype was accompanied by diminished osteoclast number and attenuated bone formation at the trabecular bone surface. Reduced RANKL signaling in calvarial MALPs abolished osteolytic lesions after lipopolysaccharide (LPS) injections. Furthermore, in ovariectomized mice, elevated bone resorption was partially attenuated by RANKL deficiency in MALPs. In summary, our studies identified MALPs as a critical player in controlling bone remodeling during normal bone metabolism and pathological bone loss in a RANKL-dependent fashion.
Paper proposed by Stephanie Lucas
Ablation of Fat Cells in Adult Mice Induces Massive Bone Gain
Cell Metabolism 32, 1–13November 3, 2020. doi: https://doi.org/10.1016/j.cmet.2020.09.011
Adipocytes control bone mass, but the mechanism is unclear. To explore the effect of postnatal adipocyte elimination on bone cells, we mated mice expressing an inducible primate diphtheria toxin receptor (DTR) to those bearing adiponectin (ADQ)-Cre. DTR activation eliminates peripheral and marrow adipocytes in these DTRADQ mice. Within 4 days of DTR activation, the systemic bone mass of DTRADQ mice began to increase due to stimulated osteogenesis, with a 1,000% expansion by 10–14 days post-DTR treatment. This adipocyte ablation-mediated enhancement of skeletal mass reflected bone morphogenetic protein (BMP) receptor activation following the elimination of its inhibitors, associated with simultaneous epidermal growth factor (EGF) receptor signaling. DTRADQ-induced osteosclerosis is not due to ablation of peripheral adipocytes but likely reflects the elimination of marrow ADQ-expressing cells. Thus, anabolic drugs targeting BMP receptor inhibitors with short-term EGF receptor activation may be a means of profoundly increasing skeletal mass to prevent or reverse pathological bone loss.
(proposed by Annegreet Veldhuis-Vlug)
Adipocytes in hematopoiesis and acute leukemia: friends, enemies, or innocent bystanders?
Julia Zinngrebe, Klaus-Michael Debatin, Pamela Fischer-Posovszky
Leukemia 34, 2305-2316 (2020) . doi:10.1038/s41375-020-0886-x
The bone marrow is home to well-balanced normal hematopoiesis, but also the stage of leukemia’s crime. Marrow adipose tissue (MAT) is a unique and versatile component of the bone marrow niche. While the importance of MAT for bone health has long been recognized, its complex role in hematopoiesis has only recently gained attention. In this review article we summarize recent conceptual advances in the field of MAT research and how these developments impact our understanding of MAT regulation of hematopoiesis. Elucidating routes of interaction and regulation between MAT and cells of the hematopoietic system are essential to pinpoint vulnerable processes resulting in malignant transformation. The concept of white adipose tissue contributing to cancer development and progression on the cellular, metabolic, and systemic level is generally accepted. The role of MAT in malignant hematopoiesis, however, is controversial. MAT is very sensitive to changes in the patient’s metabolic status hampering a clear definition of its role in different clinical situations. Here, we discuss future directions for leukemia research in the context of metabolism-induced modifications of MAT and other adipose tissues and how this might impact on leukemia cell survival, proliferation, and antileukemic therapy.
(proposed by Alessandro Corsi)
IRX3 and IRX5 inhibit adipogenic differentiation of hypertrophic chondrocytes and promote osteogenesis
Journal of Bone and Mineral Research, Vol. 00, No. 00, Month 2020, pp 1–14 doi: https://doi.org/10.1002/jbmr.4132
Maintaining the correct proportions of different cell types in the bone marrow is critical for bone function. Hypertrophic chondrocytes (HCs) and osteoblasts are a lineage continuum with a minor contribution to adipocytes, but the regulatory network is unclear. Mutations in transcription factors, IRX3 and IRX5, result in skeletal patterning defects in humans and mice. We found coexpression of Irx3 and Irx5 in late‐stage HCs and osteoblasts in cortical and trabecular bone. Irx3 and Irx5 null mutants display severe bone deficiency in newborn and adult stages. Quantitative analyses of bone with different combinations of functional alleles of Irx3 and Irx5 suggest these two factors function in a dosage‐dependent manner. In Irx3 and Irx5 nulls, the amount of bone marrow adipocytes was increased. In Irx5 nulls, lineage tracing revealed that removal of Irx3 specifically in HCs exacerbated reduction of HC‐derived osteoblasts and increased the frequency of HC‐derived marrow adipocytes. β‐catenin loss of function and gain of function specifically in HCs affects the expression of Irx3 and Irx5, suggesting IRX3 and IRX5 function downstream of WNT signaling. Our study shows that IRX3 and IRX5 regulate fate decisions in the transition of HCs to osteoblasts and to marrow adipocytes, implicating their potential roles in human skeletal homeostasis and disorders.
(proposed by Christophe Chauveau)
Human Bone Marrow Is Comprised of Adipocytes with Specific Lipid Metabolism
Camille Attané, David Estève, Karima Chaoui, Jason S.Iacovoni, Jill Corre, Mohamed Moutahir, Philippe Valet, Odile Schiltz, Nicolas Reina, Catherine Muller
Cell Reports. 2020 Jan 28;30(4):949-958. doi: https://doi.org/10.1016/j.celrep.2019.12.089
Under caloric restriction, bone marrow adipocytes (BM-Ads) do not decrease in size compared to white adipocytes, suggesting they harbor unique metabolic properties. We compare human primary BM-Ads with paired subcutaneous adipocytes (SC-Ads) using proteomic and lipidomic approaches. We find that, although SC-Ads and BM-Ads share similar morphological features, they possess distinct lipid metabolism. Although BM-Ad shows enrichment in proteins involved in cholesterol metabolism, correlating with increased free cholesterol content, proteins involved in lipolysis were downregulated. In particular, monoacylglycerol lipase expression is strongly reduced in BM-Ads, leading to monoacylglycerol accumulation. Consequently, basal and induced lipolytic responses are absent in BM-Ads, affirming their differences in metabolic fitness upon caloric restriction. These specific metabolic features are not recapitulated in vitro using common protocols to differentiate bone marrow mesenchymal stem cells. Thus, contrary to classical SC-Ads, BM-Ads display a specific lipid metabolism, as they are devoid of lipolytic activity and exhibit a cholesterol-orientated metabolism.
(proposed by Annegreet Veldhuis-Vlug)
A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration.
Yuki Matsushita, Mizuki Nagata, Kenneth M. Kozloff, Joshua D. Welch, Koji Mizuhashi, Nicha Tokavanich, Shawn A. Hallett, Daniel C. Link, Takashi Nagasawa, Wanida Ono & Noriaki Ono
Nat Commun. 2020 Jan 16;11(1):332. doi: 10.1038/s41467-019-14029-w
Bone marrow stromal cells (BMSCs) are versatile mesenchymal cell populations underpinning the major functions of the skeleton, a majority of which adjoin sinusoidal blood vessels and express C-X-C motif chemokine ligand 12 (CXCL12). However, how these cells are activated during regeneration and facilitate osteogenesis remains largely unknown. Cell-lineage analysis using Cxcl12-creER mice reveals that quiescent Cxcl12-creER+ perisinusoidal BMSCs differentiate into cortical bone osteoblasts solely during regeneration. A combined single cell RNA-seq analysis demonstrate that these cells convert their identity into a skeletal stem cell-like state in response to injury, associated with upregulation of osteoblast-signature genes and activation of canonical Wnt signaling components along the single-cell trajectory. β-catenin deficiency in these cells indeed causes insufficiency in cortical bone regeneration. Therefore, quiescent Cxcl12-creER+ BMSCs transform into osteoblast precursor cells in a manner mediated by canonical Wnt signaling, highlighting a unique mechanism by which dormant stromal cells are enlisted for skeletal regeneration.
(proposed by Michaela Reagan)
Lipid availability determines fate of skeletal progenitor cells via SOX9.
Nick van Gastel, Steve Stegen, Guy Eelen, Sandra Schoors, Aurélie Carlier, Veerle W. Daniëls, Ninib Baryawno, Dariusz Przybylski, Maarten Depypere, Pieter-Jan Stiers, Dennis Lambrechts, Riet Van Looveren, Sophie Torrekens, Azeem Sharda, Patrizia Agostinis, Diether Lambrechts, Frederik Maes, Johan V. Swinnen, Liesbet Geris, Hans Van Oosterwyck, Bernard Thienpont, Peter Carmeliet, David T. Scadden & Geert Carmeliet
Nature. 2020 Mar;579(7797):111-117. doi: 10.1038/s41586-020-2050-1. Epub 2020 Feb 26.
The avascular nature of cartilage makes it a unique tissue, but whether and how the absence of nutrient supply regulates chondrogenesis remain unknown. Here we show that obstruction of vascular invasion during bone healing favours chondrogenic over osteogenic differentiation of skeletal progenitor cells. Unexpectedly, this process is driven by a decreased availability of extracellular lipids. When lipids are scarce, skeletal progenitors activate forkhead box O (FOXO) transcription factors, which bind to the Sox9 promoter and increase its expression. Besides initiating chondrogenesis, SOX9 acts as a regulator of cellular metabolism by suppressing oxidation of fatty acids, and thus adapts the cells to an avascular life. Our results define lipid scarcity as an important determinant of chondrogenic commitment, reveal a role for FOXO transcription factors during lipid starvation, and identify SOX9 as a critical metabolic mediator. These data highlight the importance of the nutritional microenvironment in the specification of skeletal cell fate.
(proposed by Greet Kerckhofs)
Induction of Stearoyl-CoA 9-Desaturase 1 Protects Human Mesenchymal Stromal Cells Against Palmitic Acid-Induced Lipotoxicity and Inflammation.
Antoine Dalla Valle, Pascale Vertongen, Delphine Spruyt, Jessica Lechanteur, Valérie Suain, Nathalie Gaspard, Jean-Pierre Brion, Valérie Gangji and Joanne Rasschaert
Front Endocrinol (Lausanne). 2019 Oct 24;10:726. doi: 10.3389/fendo.2019.00726.
In bone diseases such as osteonecrosis and osteoporosis, a shift toward a preferential differentiation of mesenchymal stromal cells (MSC) into adipocytes at the expense of the osteoblastic lineage is described, leading to excessive accumulation of adipocytes in the bone marrow of the patients. The influence of cytokines and adipokines secreted by adipocytes on skeletal health is already well-documented but the impact of free fatty acids release on bone cell biology and viability is an emerging concept. We have previously demonstrated that the saturated fatty acid (SFA) palmitate (Palm) is cytotoxic for human MSC (hMSC) and osteoblasts whereas oleate (Ole), a monounsaturated fatty acid (MUFA), has no toxic effect. Moreover, Ole protects cells against lipotoxicity. Our observations led us to propose that the toxicity of the SFA is not correlated to its intracellular accumulation but could rather be related to the intracellular SFA/MUFA ratio, which finally determines the toxic effect of SFA. Therefore, in the present study, we have investigated the potential protective role of the enzyme stearoyl-CoA 9-desaturase 1 (SCD1) against the deleterious effects of Palm. SCD1 is an enzyme responsible for desaturation of SFA to MUFA; its activation could therefore lead to modifications of the intracellular SFA/MUFA ratio. In the present study, we showed that hMSC express SCD1 and liver X receptors (LXRs), transcription factors regulating SCD1 expression. Human MSC treatment with a LXRs agonist triggered SCD1 expression and drastically reduced Palm-induced cell mortality, caspases 3/7 activation, endoplasmic reticulum stress and inflammation. We also observed that, in the presence of Palm, the LXRs agonist provoked lipid droplets formation, augmented the total cellular neutral lipid content but decreased the SFA/MUFA ratio when compared to Palm treatment alone. Addition of an inhibitor of SCD1 activity abrogated the positive effects of the LXRs agonist, suggesting that SCD1 could play a key role in protecting hMSC against lipotoxicity.
(proposed by William Ferris)
Exercise Degrades Bone in Caloric Restriction, Despite Suppression of Marrow Adipose Tissue (MAT)
Cody McGrath, Jeyantt S Sankaran, Negin Misaghian-Xanthos, Buer Sen, Zhihui Xie, Martin A Styner, Xiaopeng Zong, Janet Rubin, Maya Styner
J Bone Miner Res. 2019 Sep 11. doi: 10.1002/jbmr.3872
Marrow adipose tissue (MAT) and its relevance to skeletal health during caloric restriction (CR) is unknown: It remains unclear whether exercise, which is anabolic to bone in a calorie-replete state, alters bone or MAT in CR. We hypothesized that response of bone and MAT to exercise in CR differs from the calorie-replete state. Ten-week-old female B6 mice fed a regular diet (RD) or 30% CR diet were allocated to sedentary (RD, CR, n = 10/group) or running exercise (RD-E, CR-E, n = 7/group). After 6 weeks, CR mice weighed 20% less than RD, p < 0.001; exercise did not affect weight. Femoral bone volume (BV) via 3D MRI was 20% lower in CR versus RD (p < 0.0001). CR was associated with decreased bone by μCT: Tb.Th was 16% less in CR versus RD, p < 0.003, Ct.Th was 5% less, p < 0.07. In CR-E, Tb.Th was 40% less than RD-E, p < 0.0001. Exercise increased Tb.Th in RD (+23% RD-E versus RD, p < 0.003) but failed to do so in CR. Cortical porosity increased after exercise in CR (+28%, p = 0.04), suggesting exercise during CR is deleterious to bone. In terms of bone fat, metaphyseal MAT/ BV rose 159% in CR versus RD, p = 0.003 via 3D MRI. Exercise decreased MAT/BV by 52% in RD, p < 0.05, and also suppressed MAT in CR (-121%, p = 0.047). Histomorphometric analysis of adipocyte area correlated with MAT by MRI (R2 = 0.6233, p < 0.0001). With respect to bone, TRAP and Sost mRNA were reduced in CR. Intriguingly, the repressed Sost in CR rose with exercise and may underlie the failure of CR-bone quantity to increase in response to exercise. Notably, CD36, a marker of fatty acid uptake, rose 4088% in CR (p < 0.01 versus RD), suggesting that basal increases in MAT during calorie restriction serve to supply local energy needs and are depleted during exercise with a negative impact on bone.
(proposed by Erica Scheller)
Glutamine Metabolism Regulates Proliferation and Lineage Allocation in Skeletal Stem Cells.
Yilin Yu, Hunter Newman, Leyao Shen, Deepika Sharma, Guoli Hu, Anthony J. Mirando, Hongyuan Zhang, Everett Knudsen, Guo-Fang Zhang, Matthew J. Hilton, Courtney M. Karner
Cell Metab. 2019 Apr 2;29(4):966-978.e4. doi: 10.1016/j.cmet.2019.01.016. Epub 2019 Feb 14
Skeletal stem cells (SSCs) are postulated to provide a continuous supply of osteoblasts throughout life. However, under certain conditions, the SSC population can become incorrectly specified or is not maintained, resulting in reduced osteoblast formation, decreased bone mass, and in severe cases, osteoporosis. Glutamine metabolism has emerged as a critical regulator of many cellular processes in diverse pathologies. The enzyme glutaminase (GLS) deaminates glutamine to form glutamate-the rate-limiting first step in glutamine metabolism. Using genetic and metabolic approaches, we demonstrate GLS and glutamine metabolism are required in SSCs to regulate osteoblast and adipocyte specification and bone formation. Mechanistically, transaminase-dependent α-ketoglutarate production is critical for the proliferation, specification, and differentiation of SSCs. Collectively, these data suggest stimulating GLS activity may provide a therapeutic approach to expand SSCs in aged individuals and enhance osteoblast differentiation and activity to increase bone mass.
(proposed by Beata Lecka-Czernik)
Proton Density Fat Fraction MRI of Vertebral Bone Marrow: Accuracy, Repeatability, and Reproducibility Among Readers, Field Strengths, and Imaging Platforms
Frederic Carsten Schmeel, Toni Vomweg, Frank Träber, Arnd Gerhards, Simon Jonas Enkirch, Anton Faron, Alois Martin Sprinkart, Leonard Christopher Schmeel, Julian Alexander Luetkens, Daniel Thomas, and Guido Matthias Kukuk
J Magn Reson Imaging. 2019 Apr 13. doi: 10.1002/jmri.26748.
BACKGROUND: Chemical shift-encoding based water-fat MRI is an emerging method to noninvasively assess proton density fat fraction (PDFF), a promising quantitative imaging biomarker for estimating tissue fat concentration. However, in vivo validation of PDFF is still lacking for bone marrow applications.
PURPOSE: To determine the accuracy and precision of MRI-determined vertebral bone marrow PDFF among different readers and across different field strengths and imager manufacturers.
STUDY TYPE: Repeatability/reproducibility.
SUBJECTS: Twenty-four adult volunteers underwent lumbar spine MRI with one 1.5T and two different 3.0T MR scanners from two vendors on the same day.
FIELD STRENGTH/SEQUENCE: 1.5T and 3.0T/3D spoiled-gradient echo multipoint Dixon sequences.
ASSESSMENT: Two independent readers measured intravertebral PDFF for the three most central slices of the L1-5 vertebral bodies. Single-voxel MR spectroscopy (MRS)-determined PDFF served as the reference standard for PDFF estimation.
STATISTICAL TESTS: Accuracy and bias were assessed by Pearson correlation, linear regression analysis, and Bland-Altman plots. Repeatability and reproducibility were evaluated by Wilcoxon signed rank test, Friedman test, and coefficients of variation. Intraclass correlation coefficients were used to validate intra- and interreader as well as intraimager agreements.
RESULTS: MRI-based PDFF estimates of lumbar bone marrow were highly correlated (r2 = 0.899) and accurate (mean bias, -0.6%) against the MRS-determined PDFF reference standard. PDFF showed high linearity (r2 = 0.972-0.978) and small mean bias (0.6-1.5%) with 95% limits of agreement within ±3.4% across field strengths, imaging platforms, and readers. Repeatability and reproducibility of PDFF were high, with the mean overall coefficient of variation being 0.86% and 2.77%, respectively. The overall intraclass correlation coefficient was 0.986 as a measure for an excellent interreader agreement.
DATA CONCLUSION: MRI-based quantification of vertebral bone marrow PDFF is highly accurate, repeatable, and reproducible among readers, field strengths, and MRI platforms, indicating its robustness as a quantitative imaging biomarker for multicentric studies.
LEVEL OF EVIDENCE: 3 Technical Efficacy: Stage 2 J. Magn. Reson. Imaging 2019.
(proposed by Dimitrios Karampinos)
Adding Marrow Adiposity and Cortical Porosity to Femoral Neck Areal Bone Mineral Density Improves the Discrimination of Women with Nonvertebral Fractures from Controls.
Roger Zebaze, Marit Osima, Minh Bui, Marko Lukic, Xiaofang Wang, Ali Ghasem‐Zadeh, Erik F. Eriksen, Angela Vais, Catherine Shore‐Lorenti, Peter Ebeling, Ego Seeman, Åshild Bjørnerem
J Bone Miner Res. 2019 Mar 18. doi: 10.1002/jbmr.3721.
Advancing age is accompanied by a reduction in bone formation and remodeling imbalance, which produces microstructural deterioration. This may be partly due to diversion of mesenchymal cells towards adipocytes rather than osteoblast lineage cells. We hypothesized that microstructural deterioration will be associated with an increased marrow adiposity, and each of these traits will be independently associated with nonvertebral fractures and improve discrimination of women with fractures from controls over that achieved by femoral neck (FN) areal bone mineral density (aBMD) alone. The marrow adiposity and bone microstructure were quantified from high-resolution peripheral quantitative computed tomography (HR-pQCT) images of the distal tibia and distal radius in 77 women aged 40-70 years with a recent nonvertebral fracture and 226 controls in Melbourne, Australia. Marrow fat measurement from HR-pQCT images was validated using direct histologic measurement as gold standard, at the distal radius of 15 sheep, with an agreement (R2 = 0.86, p < 0.0001). Each standard deviation (SD) higher distal tibia marrow adiposity was associated with 0.33 SD higher cortical porosity, 0.60 SD fewer, 0.24 SD thinner and 0.72 SD more separated trabeculae (all p < 0.05). Adjusted for age and FN aBMD, odds ratios (95% confidence interval) for fracture per SD higher marrow adiposity and cortical porosity were 3.39 (2.14-5.38) and 1.79 (1.14-2.80), respectively. Discrimination of women with fracture from controls improved when cortical porosity was added to FN aBMD and age (AUC 0.778 vs. 0.751, p = 0.006) or marrow adiposity was added to FN aBMD and age (AUC 0.825 vs. 0.751, p = 0.002). The model including FN aBMD, age, cortical porosity, trabecular thickness and marrow adiposity had an AUC = 0.888. Results were similar for the distal radius. Whether marrow adiposity and cortical porosity indices improve identification of women at risk for fractures requires validation in pro
(proposed by Guillaume Penel)
Osteogenesis depends on commissioning of a network of stem cell transcription factors that act as repressors of adipogenesis
Alexander Rauch, Anders K. Haakonsson, Jesper G. S. Madsen, Mette Larsen, Isabel Forss, Martin R. Madsen, Elvira L. Van Hauwaert, Christian Wiwie, Naja Z. Jespersen, Michaela Tencerova, Ronni Nielsen, Bjørk D. Larsen, Richard Röttger, Jan Baumbach, Camilla Scheele, Moustapha Kassem and Susanne Mandrup
Nat Genet. 2019 Apr;51(4):716-727. doi: 10.1038/s41588-019-0359-1.
Mesenchymal (stromal) stem cells (MSCs) constitute populations of mesodermal multipotent cells involved in tissue regeneration and homeostasis in many different organs. Here we performed comprehensive characterization of the transcriptional and epigenomic changes associated with osteoblast and adipocyte differentiation of human MSCs. We demonstrate that adipogenesis is driven by considerable remodeling of the chromatin landscape and de novo activation of enhancers, whereas osteogenesis involves activation of preestablished enhancers. Using machine learning algorithms for in silico modeling of transcriptional regulation, we identify a large and diverse transcriptional network of pro-osteogenic and antiadipogenic transcription factors. Intriguingly, binding motifs for these factors overlap with SNPs related to bone and fat formation in humans, and knockdown of single members of this network is sufficient to modulate differentiation in both directions, thus indicating that lineage determination is a delicate balance between the activities of many different transcription factors.
Chronic Kidney Disease Is Associated With Greater Bone Marrow Adiposity
Gina N Woods, Susan K Ewing, Sigurdur Sigurdsson, Deborah M Kado, Joachim H Ix,Trisha F Hue, Gudny Eiriksdottir, Kaipin Xu, Vilmundur Gudnason, Thomas F Lang, Eric Vittinghoff, Tamara B Harris, Clifford J Rosen, Xiaojuan Li, Ann V Schwartz. J Bone Miner Res.2018 Aug 3. doi: 10.1002/jbmr.3562
Bone marrow adiposity is associated with aging, osteoporosis, and reduced hematopoiesis, as well as anorexia nervosa, but little is known about the underlying mechanisms that affect marrow adiposity. Chronic kidney disease (CKD) may influence bone marrow adipose tissue (BMAT), possibly through loss of lean mass or higher circulating levels of sclerostin. To test these hypotheses, we investigated the cross‐sectional association between estimated glomerular filtration rate (eGFR) as a measure of kidney function and 1H‐MRS‐based measurement of vertebral BMAT (L1 to L4) in 475 older adults from the Age Gene/Environment Susceptibility (AGES)‐Reykjavik study. Mean BMAT was compared in those with eGFR >60 (n = 297) versus those with eGFR 45 to 60 (n = 120) or eGFR <45 (n = 58) using linear regression models. Participants had a mean age of 81.5 (SD 4.1) years, mean eGFR of 64.3 (SD 16.1) mL/min/1.734 cm2, mean BMAT of 54.5% (SD 8.5); 48.2% were women. In unadjusted and adjusted models (age, visit window, gender, diabetes and visceral adipose tissue), BMAT was higher in those with eGFR <45 (adjusted mean 58.5%; 95% CI, 56.2 to 60.7) compared with those with eGFR >60 (adjusted mean 53.8%; 95% CI, 52.8 to 54.8) (p = 0.0002). BMAT did not differ in those with eGFR 45 to 60 (adjusted mean 54.3%; 95% CI, 52.8 to 55.9) compared with those with eGFR >60 (p = 0.58). In a subgroup of participants with serum sclerostin available (n = 253), additional adjustment for sclerostin attenuated the difference in adjusted mean vertebral BMAT between those with eGFR <45 versus >60 from 3.7% (p = 0.04) to 2.4% (p = 0.20). CKD stage 3b or worse was associated with greater bone marrow adiposity; this association may be partially mediated by sclerostin.
Specific Modulation of Vertebral Marrow Adipose Tissue by Physical Activity
Daniel L Belavy, Matthew J Quittner, Nicola D Ridgers, Adnan Shiekh, Timo Rantalainen,Guy Trudel. J Bone Miner Res. 2018 Apr;33(4):651-657. doi: 10.1002/jbmr.3357. Epub 2018 Jan 16.
Marrow adipose tissue (MAT) accumulation with normal aging impacts the bone, hemopoiesis, and metabolic pathways. We investigated whether exercise was associated with lower MAT, as measured by vertebral marrow fat fraction (VFF) on magnetic resonance imaging. A total of 101 healthy individuals (54 females) aged 25 to 35 years without spine or bone disease but with distinct exercise histories were studied. Long-distance runners (67 km/wk, n = 25) exhibited lower mean lumbar VFF (27.9% [8.6%] versus 33.5% [6.0%]; p = 0.0048) than non-sporting referents (n = 24). In habitual joggers (28 km/wk, n = 30), mean lumbar VFF was 31.3% (9.0%) (p = 0.22 versus referents) and 6.0 percentage points lower than referents at vertebrae T10 , T11 , and T12 (p ≤ 0.023). High-volume road cycling (275 km/wk, n = 22) did not impact VFF. 3D accelerations corresponding to faster walking, slow jogging, and high-impact activities correlated with lower VFF, whereas low-impact activities and sedentary time correlated with higher mean lumbar VFF (all p ≤ 0.05). Given an estimated adipose bone marrow conversion of 7% per decade of life, long distance runners, with 5.6 percentage points lower VFF, showed an estimated 8-year younger vertebral marrow adipose tissue phenotype. Regression analysis showed a 0.7 percentage point reduction in mean lumbar VFF with every 9.4 km/wk run (p = 0.002). This study presents the first evidence in humans or animals that specific volumes and types of exercise may influence the age-determined adipose marrow conversion and result in low MAT. These results identify a potentially modifiable risk factor for prevalent chronic conditions related to bone metabolism, hemopoietic production, and other metabolic functions with potential global health applications.
Development of a 3D bone marrow adipose tissue model.
Heather Fairfield, Carolyne Falank, Mariah Farrell, CalvinVary, Joshua M. Boucher, HeatherDriscoll, Lucy Liaw, Clifford J. Rosen, Michaela R. Reagan. Bone. 2018 Jan 26. pii: S8756-3282(18)30023-1. doi: 10.1016/j.bone.2018.01.023.
Over the past twenty years, evidence has accumulated that biochemically and spatially defined networks of extracellular matrix, cellular components, and interactions dictate cellular differentiation, proliferation, and function in a variety of tissue and diseases. Modeling in vivo systems in vitro has been undeniably necessary, but when simplified 2D conditions rather than 3D in vitro models are used, the reliability and usefulness of the data derived from these models decreases. Thus, there is a pressing need to develop and validate reliable in vitro models to reproduce specific tissue-like structures and mimic functions and responses of cells in a more realistic manner for both drug screening/disease modeling and tissue regeneration applications. In adipose biology and cancer research, these models serve as physiologically relevant 3D platforms to bridge the divide between 2D cultures and in vivo models, bringing about more reliable and translationally useful data to accelerate benchtop to bedside research. Currently, no model has been developed for bone marrow adipose tissue (BMAT), a novel adipose depot that has previously been overlooked as “filler tissue” but has more recently been recognized as endocrine-signaling and systemically relevant. Herein we describe the development of the first 3D, BMAT model derived from either human or mouse bone marrow (BM) mesenchymal stromal cells (MSCs). We found that BMAT models can be stably cultured for at least 3 months in vitro, and that myeloma cells (5TGM1, OPM2 and MM1S cells) can be cultured on these for at least 2 weeks. Upon tumor cell co-culture, delipidation occurred in BMAT adipocytes, suggesting a bidirectional relationship between these two important cell types in the malignant BM niche. Overall, our studies suggest that 3D BMAT represents a “healthier,” more realistic tissue model that may be useful for elucidating the effects of MAT on tumor cells, and tumor cells on MAT, to identify novel therapeutic targets. In addition, proteomic characterization as well as microarray data (expression of >22,000 genes) coupled with KEGG pathway analysis and gene set expression analysis (GSEA) supported our development of less-inflammatory 3D BMAT compared to 2D culture. In sum, we developed the first 3D, tissue-engineered bone marrow adipose tissue model, which is a versatile, novel model that can be used to study numerous diseases and biological processes involved with the bone marrow.
Characterization of the bone marrow adipocyte niche with three-dimensional electron microscopy
Hero Robles, Sung Jae Park, Matthew S. Joens, James A. J. Fitzpatrick, Clarissa S. Craft, Erica L. Scheller. Bone. 2018 Jan 27. pii: S8756-3282(18)30020-6. doi: 10.1016/j.bone.2018.01.020.
Unlike white and brown adipose tissues, the bone marrow adipocyte (BMA) exists in a microenvironment containing unique populations of hematopoietic and skeletal cells. To study this microenvironment at the sub-cellular level, we performed a three-dimensional analysis of the ultrastructure of the BMA niche with focused ion beam scanning electron microscopy (FIB-SEM). This revealed that BMAs display hallmarks of metabolically active cells including polarized lipid deposits, a dense mitochondrial network, and areas of endoplasmic reticulum. The distinct orientations of the triacylglycerol droplets suggest that fatty acids are taken up and/or released in three key areas – at the endothelial interface, into the hematopoietic milieu, and at the bone surface. Near the sinusoidal vasculature, endothelial cells send finger-like projections into the surface of the BMA which terminate near regions of lipid within the BMA cytoplasm. In some regions, perivascular cells encase the BMA with their flattened cellular projections, limiting contacts with other cells in the niche. In the hematopoietic milieu, BMAT adipocytes of the proximal tibia interact extensively with maturing cells of the myeloid/granulocyte lineage. Associations with erythroblast islands are also prominent. At the bone surface, the BMA extends organelle and lipid-rich cytoplasmic regions toward areas of active osteoblasts. This suggests that the BMA may serve to partition nutrient utilization between diverse cellular compartments, serving as an energy-rich hub of the stromal-reticular network. Lastly, though immuno-EM, we’ve identified a subset of bone marrow adipocytes that are innervated by the sympathetic nervous system, providing an additional mechanism for regulation of the BMA. In summary, this work reveals that the bone marrow adipocyte is a dynamic cell with substantial capacity for interactions with the diverse components of its surrounding microenvironment. These local interactions likely contribute to its unique regulation relative to peripheral adipose tissues.
Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche
Allison L. Boyd, Jennifer C. Reid, Kyle R. Salci, Lili Aslostovar, Yannick D. Benoit, Zoya, Mio Nakanishi, Deanna P. Porras, Mohammed Almakadi, Clinton J. V. Campbell, Michael F. , Catherine A. Ross, Ronan Foley, Brian Leber, David S. Allan, Mitchell Sabloff, Anargyros Xenocostas, Tony J. Collins and Mickie Bhatia. Nat Cell Biol. 2017 Nov;19(11):1336-1347. doi: 10.1038/ncb3625. Epub 2017 Oct 16
Acute myeloid leukaemia (AML) is distinguished by the generation of dysfunctional leukaemic blasts, and patients characteristically suffer from fatal infections and anaemia due to insufficient normal myelo-erythropoiesis. Direct physical crowding of bone marrow (BM) by accumulating leukaemic cells does not fully account for this haematopoietic failure. Here, analyses from AML patients were applied to both in vitro co-culture platforms and in vivo xenograft modelling, revealing that human AML disease specifically disrupts the adipocytic niche in BM. Leukaemic suppression of BM adipocytes led to imbalanced regulation of endogenous haematopoietic stem and progenitor cells, resulting in impaired myelo-erythroid maturation. In vivo administration of PPARγ agonists induced BM adipogenesis, which rescued healthy haematopoietic maturation while repressing leukaemic growth. Our study identifies a previously unappreciated axis between BM adipogenesis and normal myelo-erythroid maturation that is therapeutically accessible to improve symptoms of BM failure in AML via non-cell autonomous targeting of the niche.
Clinical Implications of Bone Marrow Adiposity.
Veldhuis-Vlug AG, Rosen CJ J Intern Med. 2017 Dec 6. doi: 10.1111/joim.12718.
Marrow adipocytes, collectively termed marrow adipose tissue (MAT), reside in the bone marrow in close contact to bone cells and hematopoietic cells. Marrow adipocytes arise from the mesenchymal stem cell and share their origin with the osteoblastst. Shifts in the lineage allocation of the mesenchymal stromal cell could potentially explain the association between increased MAT and increased fracture risk in diseases such as postmenopausal osteoporosis, anorexia nervosa and diabetes. Functionally, marrow adipocytes secrete adipokines, such as adiponectin, and cytokines, such as RANK-ligand and stem cell factor. These mediators can influence both boneremodeling and hematopoiesis by promoting bone resorption and hematopoietic recovery following chemotherapy. In addition, marrowadipocytes can secrete free fatty acids, acting as a energy supply for bone and hematopoietic cells. However, this induced lipolysis is also used by neoplastic cells to promote survival and proliferation. Therefore, MAT could represent a new therapeutic target for multiple diseases from osteoporosis to leukemia, although the exaxt characteristics and role of the marrow adipocyte in health and diseases remains to be determined. This article is protected by copyright. All rights reserved.
Bone Marrow Fat Changes After Gastric Bypass Surgery Are Associated With Loss of Bone Mass.
Kim TY, Schwartz AV, Li X, Xu K, Black DM, Petrenko DM, Stewart L, Rogers SJ, Posselt AM, Carter JT, Shoback DM, Schafer A J Bone Miner Res. 2017 Nov;32(11):2239-2247. doi: 10.1002/jbmr.3212. Epub 2017 Aug 9.
Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF.
Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ Nat Cell Biol. 2017 Aug;19(8):891-903. doi: 10.1038/ncb3570. Epub 2017 Jul 17.
Endothelial cells and leptin receptor+ (LepR+) stromal cells are critical sources of haematopoietic stem cell (HSC) niche factors, including stem cell factor (SCF), in bone marrow. After irradiation or chemotherapy, these cells are depleted while adipocytes become abundant. We discovered that bone marrow adipocytes synthesize SCF. They arise from Adipoq-Cre/ER+ progenitors, which represent ∼5% of LepR+ cells, and proliferate after irradiation. Scf deletion using Adipoq-Cre/ER inhibited haematopoietic regeneration after irradiation or 5-fluorouracil treatment, depleting HSCs and reducing mouse survival. Scf from LepR+ cells, but not endothelial, haematopoietic or osteoblastic cells, also promoted regeneration. In non-irradiated mice, Scf deletion using Adipoq-Cre/ER did not affect HSC frequency in long bones, which have few adipocytes, but depleted HSCs in tail vertebrae, which have abundant adipocytes. A-ZIP/F1 ‘fatless’ mice exhibited delayed haematopoietic regeneration in long bones but not in tail vertebrae, where adipocytes inhibited vascularization. Adipocytes are a niche component that promotes haematopoietic regeneration.
Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration.
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A, Saraiva LR, Schulz TJ. Cell Stem Cell. 2017 Jun 1;20(6):771-784.e6. doi: 10.1016/j.stem.2017.02.009. Epub 2017 Mar 16.
Aging and obesity induce ectopic adipocyte accumulation in bone marrow cavities. This process is thought to impair osteogenic and hematopoietic regeneration. Here we specify the cellular identities of the adipogenic and osteogenic lineages of the bone. While aging impairs the osteogenic lineage, high-fat diet feeding activates expansion of the adipogenic lineage, an effect that is significantly enhanced in aged animals. We further describe a mesenchymal sub-population with stem cell-like characteristics that gives rise to both lineages and, at the same time, acts as a principal component of the hematopoietic niche by promoting competitive repopulation following lethal irradiation. Conversely, bone-resident cells committed to the adipocytic lineage inhibit hematopoiesis and bone healing, potentially by producing excessive amounts of Dipeptidyl peptidase-4, a protease that is a target of diabetes therapies. These studies delineate the molecular identity of the bone-resident adipocytic lineage, and they establish its involvement in age-dependent dysfunction of bone and hematopoietic regeneration.